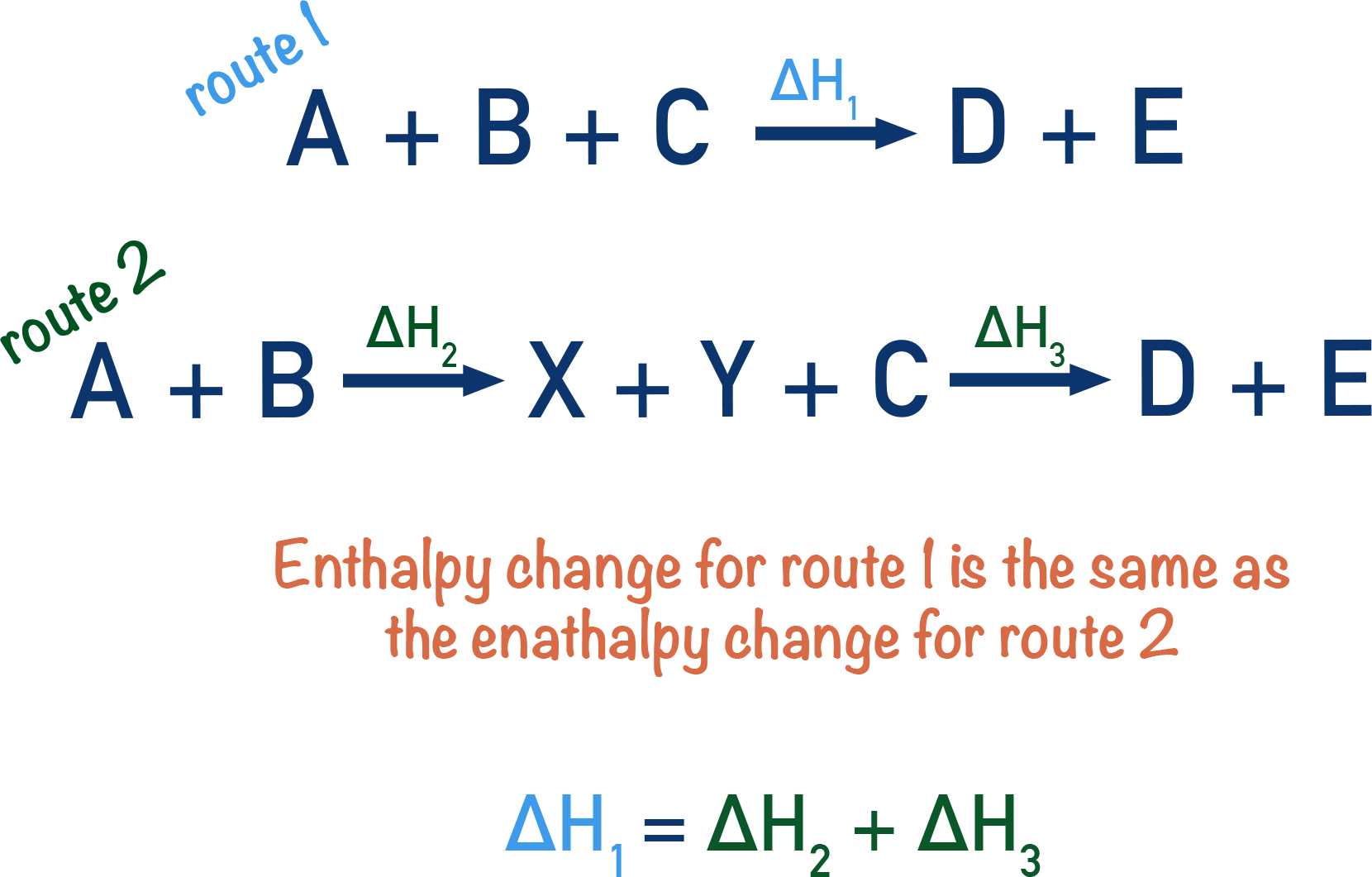Hess's Law Khan Academy
Hess's Law Khan Academy - We'll get right to the point: Start practicing—and saving your progress—now:. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall. Using hess's law and standard heats of formation to determine the enthalpy change for reactions You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and how to use. We're a nonprofit that relies on support from people like you. We're asking you to help support khan academy. You’ll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess’s law and how to use. Courses on khan academy are always 100% free. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the.
We'll get right to the point: Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the. You’ll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess’s law and how to use. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall. Using hess's law and standard heats of formation to determine the enthalpy change for reactions We're a nonprofit that relies on support from people like you. We're asking you to help support khan academy. Courses on khan academy are always 100% free. Start practicing—and saving your progress—now:. You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and how to use.
We're asking you to help support khan academy. Start practicing—and saving your progress—now:. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the. Using hess's law and standard heats of formation to determine the enthalpy change for reactions You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and how to use. We'll get right to the point: Courses on khan academy are always 100% free. We're a nonprofit that relies on support from people like you. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall. You’ll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess’s law and how to use.
Hess's LawDefinition, Application, And Examples
Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the. We're a nonprofit that relies on support from people like you. You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and.
10+ Hess Law Diagram ShaquelleLuma
Start practicing—and saving your progress—now:. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the. You'll get the quickest.
What is Hess's Law of Constant Heat Summation in 2022 Chemical
Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the. We're a nonprofit that relies on support from people like you. Start practicing—and saving your progress—now:. Courses on khan academy are always 100% free. Using hess's law and standard heats of formation to.
Hess's law and reaction enthalpy change Chemistry Khan Academy
You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and how to use. You’ll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess’s law and how to use. Hess's law states that if a process can be expressed.
Hess's law example Thermodynamics Chemistry Khan Academy YouTube
Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall. Courses on khan academy are always 100% free. You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and how to use. Hess's law states.
Hess's Law Explained Cambridge AL Chemistry YouTube
Courses on khan academy are always 100% free. You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and how to use. Start practicing—and saving your progress—now:. Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for.
Hess's LawDefinition, Application, And Examples
We'll get right to the point: We're a nonprofit that relies on support from people like you. We're asking you to help support khan academy. Using hess's law and standard heats of formation to determine the enthalpy change for reactions Start practicing—and saving your progress—now:.
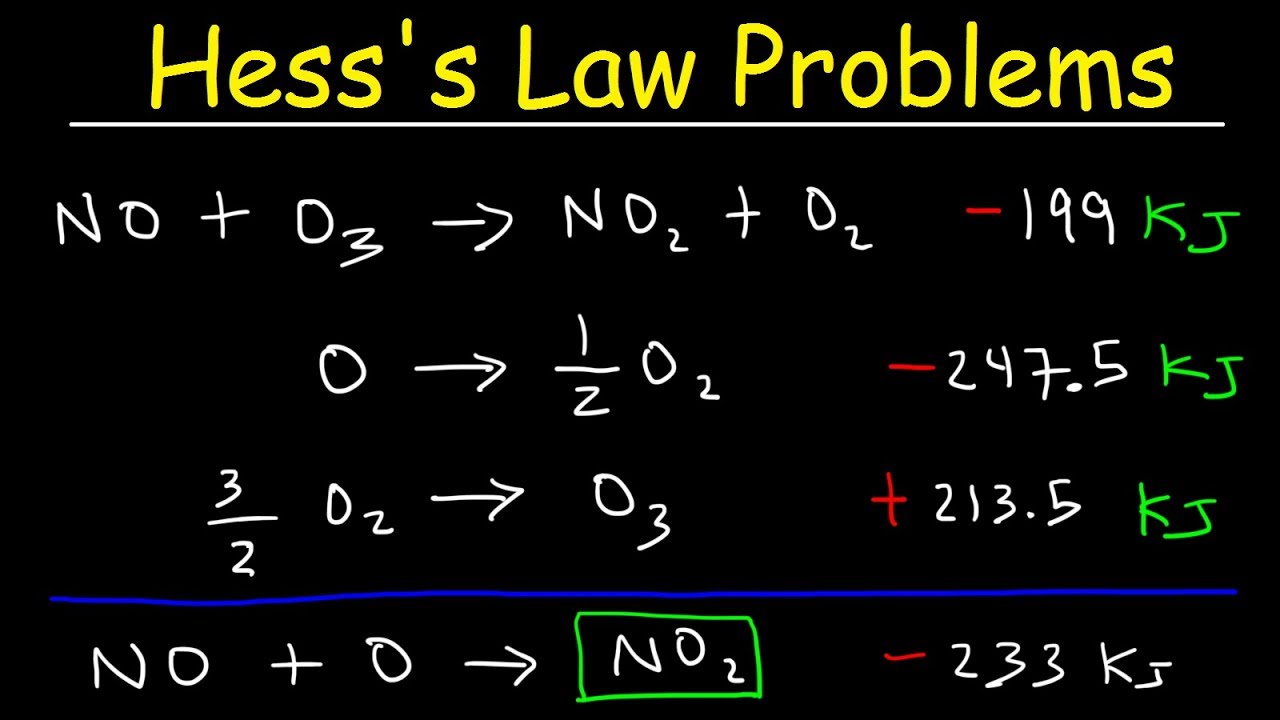
Hess's Law Problems & Enthalpy Change Chemistry YouTube
Start practicing—and saving your progress—now:. We'll get right to the point: Using hess's law and standard heats of formation to determine the enthalpy change for reactions Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the. You'll get the quickest introduction to calorimetry.
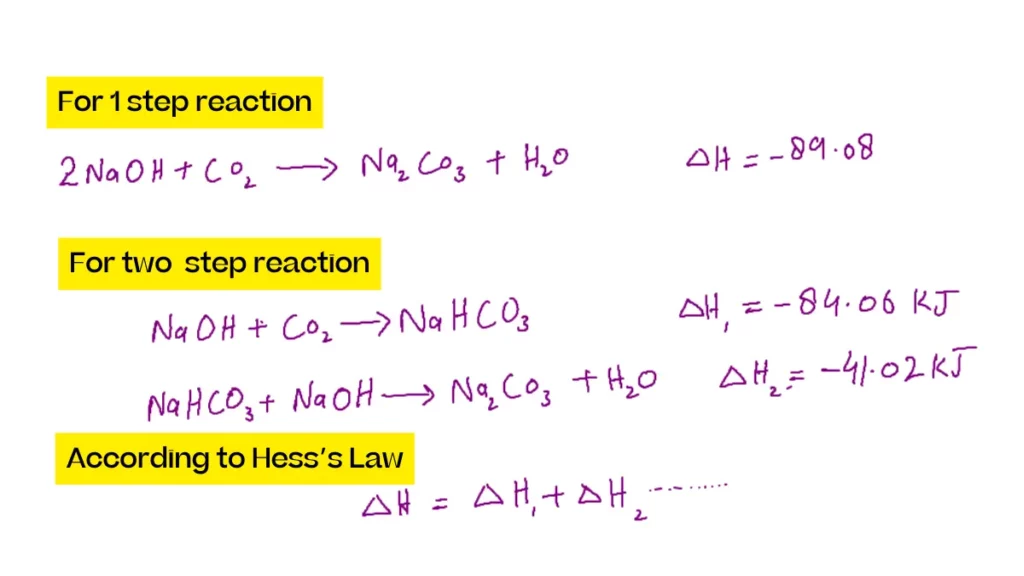
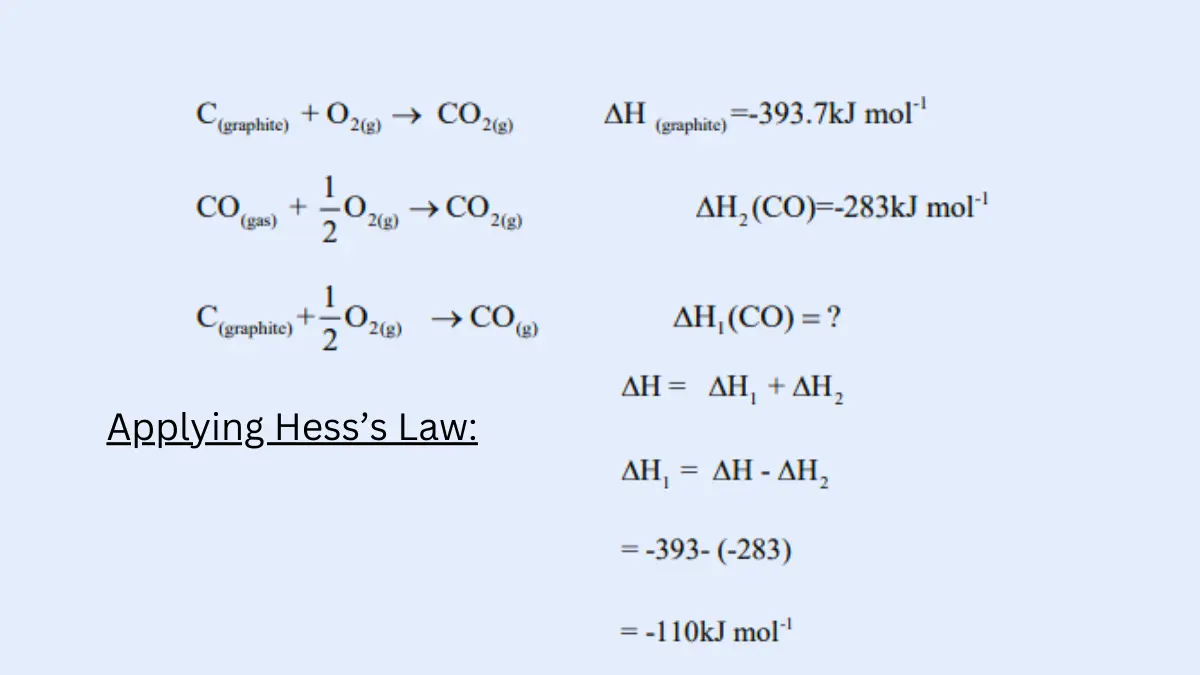
Hess s Law worked problems Hess’s Law Problems Find the ΔH for the
We're asking you to help support khan academy. You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess's law and how to use. We'll get right to the point: Start practicing—and saving your progress—now:. We're a nonprofit that relies on support from people like you.
Hess Law Calculator With Steps
We're a nonprofit that relies on support from people like you. We're asking you to help support khan academy. Using hess's law and standard heats of formation to determine the enthalpy change for reactions Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall. You'll get.
You'll Get The Quickest Introduction To Calorimetry Ever (More On That In Upcoming Episodes) And Learn The Power Of Hess's Law And How To Use.
Start practicing—and saving your progress—now:. We're a nonprofit that relies on support from people like you. You’ll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of hess’s law and how to use. Using hess's law and standard heats of formation to determine the enthalpy change for reactions
Hess's Law States That If A Process Can Be Expressed As The Sum Of Two Or More Steps, The Enthalpy Change For The Overall Process Is The.
Hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall. We're asking you to help support khan academy. Courses on khan academy are always 100% free. We'll get right to the point: