Can Carbon Form Polar And Nonpolar Bonds
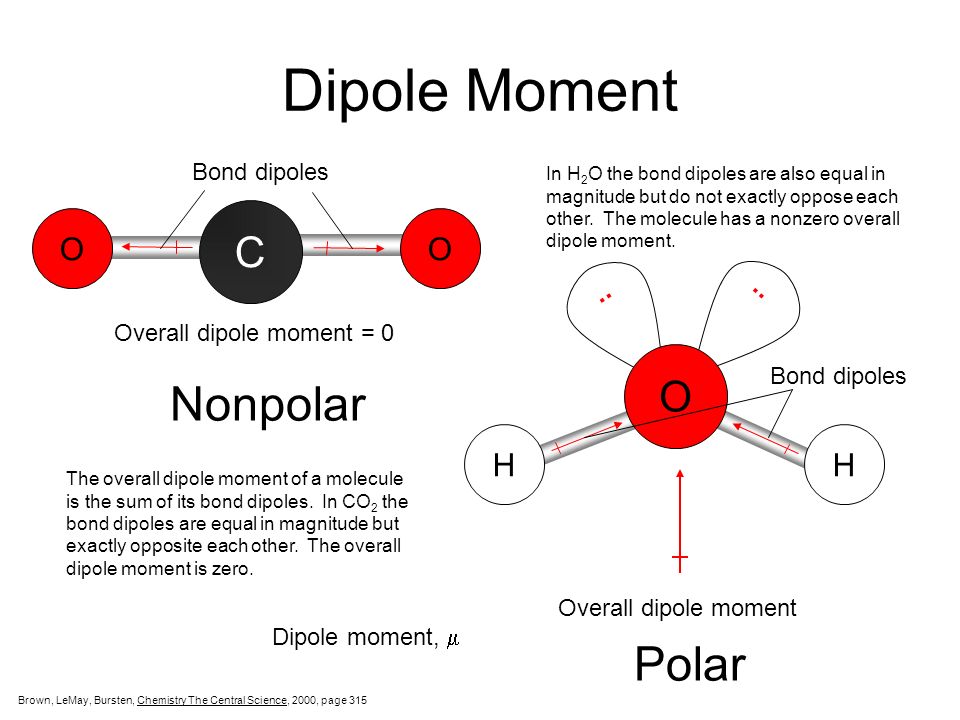
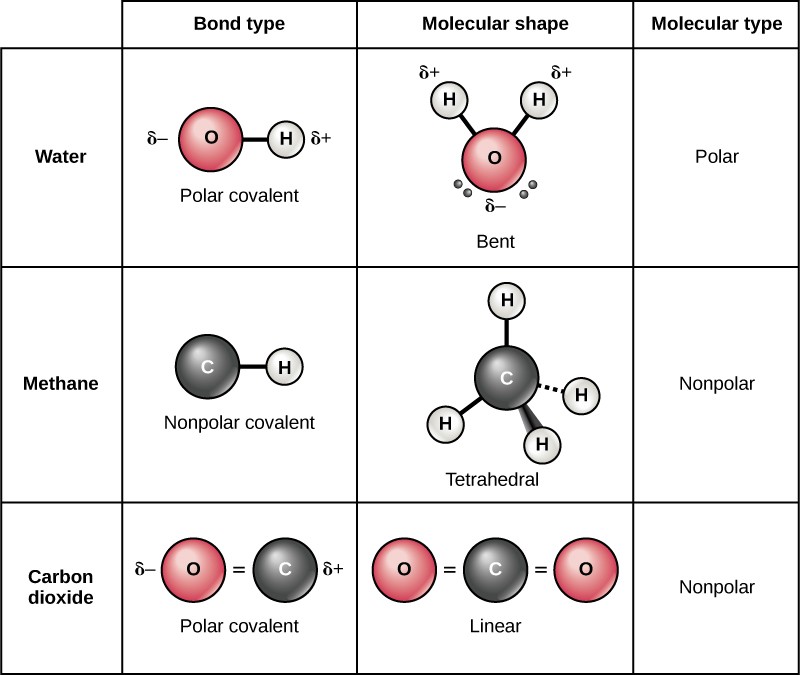
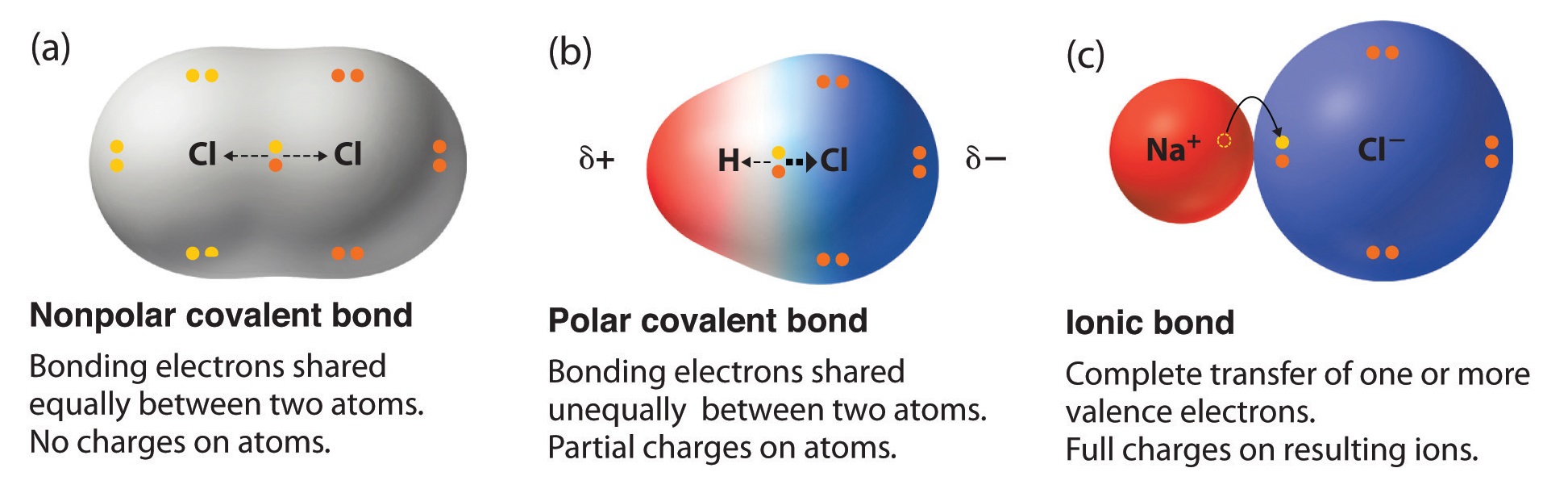
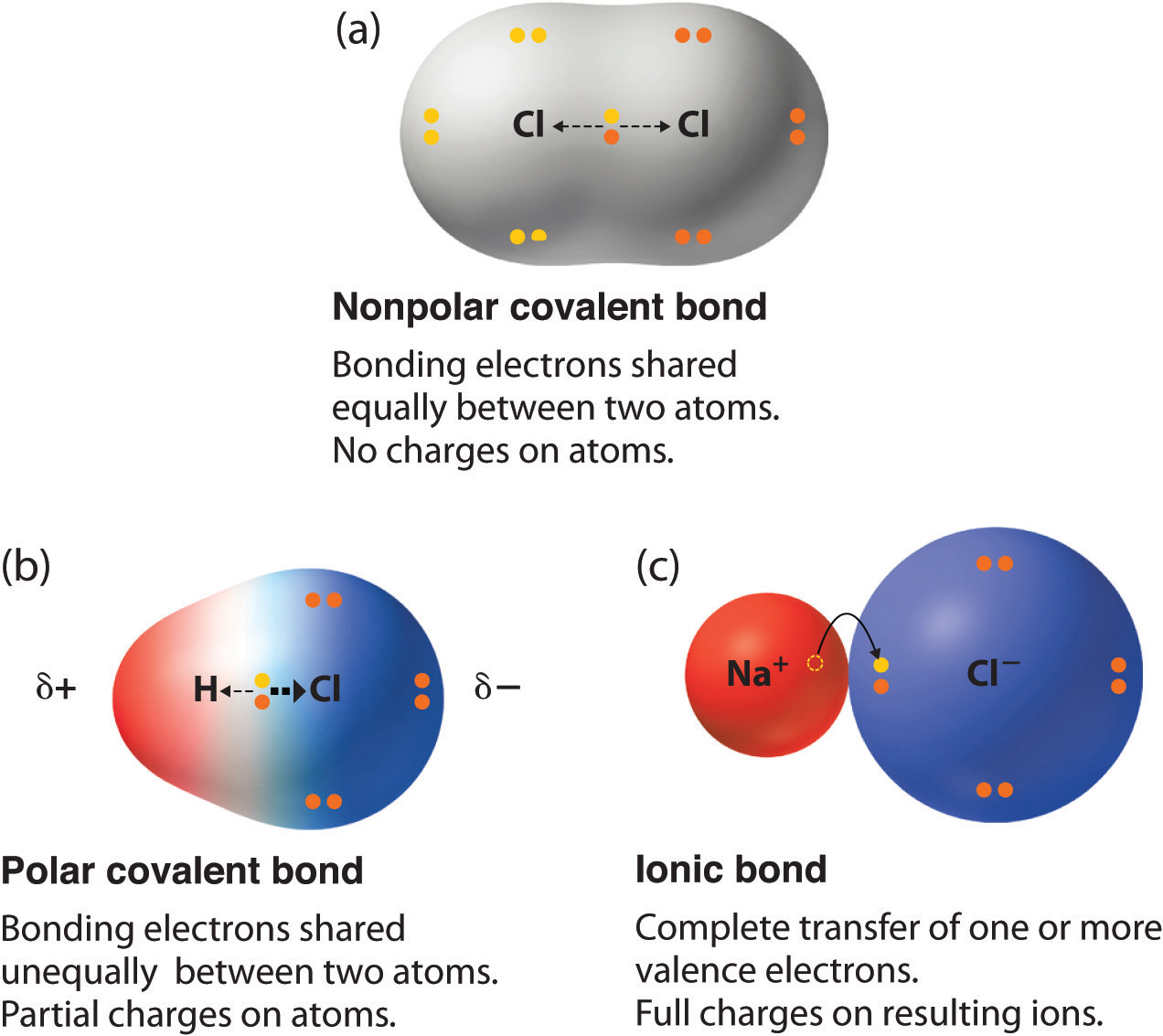
Can Carbon Form Polar And Nonpolar Bonds - Carbon bonds are stable across a broad range of temperatures. Can carbon form polar bonds? In fact, carbon is known to form polar bonds with a wide variety. Single or multiple bonds between carbon atoms are nonpolar. Is a carbon carbon bond polar or nonpolar? Carbon can form both polar and nonpolar bonds. Yes, carbon can form polar bonds. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond.
Carbon bonds are stable across a broad range of temperatures. Single or multiple bonds between carbon atoms are nonpolar. In fact, carbon is known to form polar bonds with a wide variety. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Is a carbon carbon bond polar or nonpolar? Carbon can form both polar and nonpolar bonds. Can carbon form polar bonds? Yes, carbon can form polar bonds.
Yes, carbon can form polar bonds. Single or multiple bonds between carbon atoms are nonpolar. Carbon bonds are stable across a broad range of temperatures. Carbon can form both polar and nonpolar bonds. Is a carbon carbon bond polar or nonpolar? Can carbon form polar bonds? Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. In fact, carbon is known to form polar bonds with a wide variety.
Polar vs. Nonpolar Bonds — Overview & Examples Expii Chemistry
Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Is a carbon carbon bond polar or nonpolar? In fact, carbon is known to form polar bonds with a wide variety. Single or multiple bonds between carbon atoms are nonpolar. Carbon can form both polar and nonpolar bonds.
Definition and Examples of a Polar Bond
In fact, carbon is known to form polar bonds with a wide variety. Is a carbon carbon bond polar or nonpolar? Carbon bonds are stable across a broad range of temperatures. Carbon can form both polar and nonpolar bonds. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond.
De este modo Resignación borde can carbon form polar and nonpolar bonds
Single or multiple bonds between carbon atoms are nonpolar. Carbon can form both polar and nonpolar bonds. Can carbon form polar bonds? Yes, carbon can form polar bonds. Is a carbon carbon bond polar or nonpolar?
Polar vs. Nonpolar Bonds — Overview & Examples Expii Chemistry
In fact, carbon is known to form polar bonds with a wide variety. Is a carbon carbon bond polar or nonpolar? Yes, carbon can form polar bonds. Single or multiple bonds between carbon atoms are nonpolar. Carbon bonds are stable across a broad range of temperatures.
Polar Molecule CHEMISTRY COMMUNITY
Carbon bonds are stable across a broad range of temperatures. Carbon can form both polar and nonpolar bonds. Single or multiple bonds between carbon atoms are nonpolar. In fact, carbon is known to form polar bonds with a wide variety. Yes, carbon can form polar bonds.
Chapter 2. The Chemical Context of Life Introduction to Molecular and
In fact, carbon is known to form polar bonds with a wide variety. Carbon can form both polar and nonpolar bonds. Carbon bonds are stable across a broad range of temperatures. Is a carbon carbon bond polar or nonpolar? Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond.
Which Describes Bonding Electrons in a Polar Covalent Bond JakekruwBauer
Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Yes, carbon can form polar bonds. Single or multiple bonds between carbon atoms are nonpolar. Is a carbon carbon bond polar or nonpolar? Carbon can form both polar and nonpolar bonds.
Ms J's Chemistry Class Polar vs NonPolar Covalent Bonds
In fact, carbon is known to form polar bonds with a wide variety. Single or multiple bonds between carbon atoms are nonpolar. Carbon can form both polar and nonpolar bonds. Is a carbon carbon bond polar or nonpolar? Yes, carbon can form polar bonds.
2.1 Covalent Bonds Biology LibreTexts
In fact, carbon is known to form polar bonds with a wide variety. Is a carbon carbon bond polar or nonpolar? Can carbon form polar bonds? Single or multiple bonds between carbon atoms are nonpolar. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond.
Yes, Carbon Can Form Polar Bonds.
Single or multiple bonds between carbon atoms are nonpolar. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. In fact, carbon is known to form polar bonds with a wide variety. Can carbon form polar bonds?
Carbon Bonds Are Stable Across A Broad Range Of Temperatures.
Carbon can form both polar and nonpolar bonds. Is a carbon carbon bond polar or nonpolar?

:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)