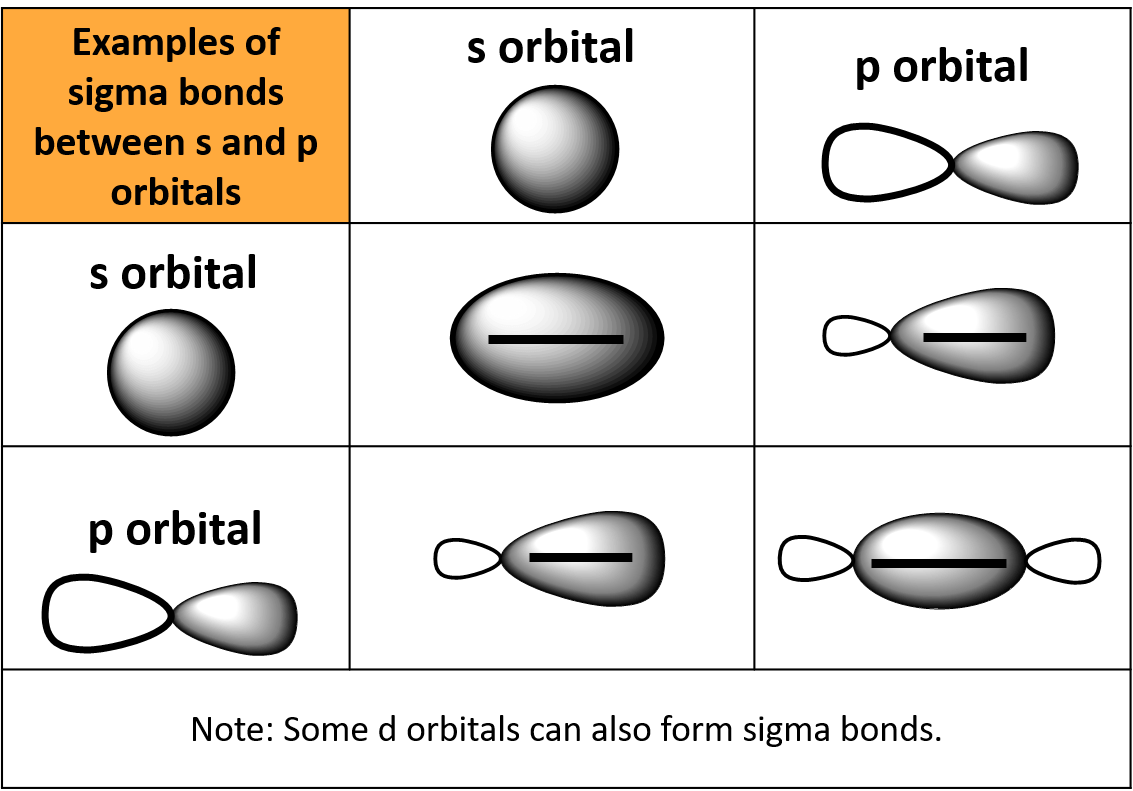
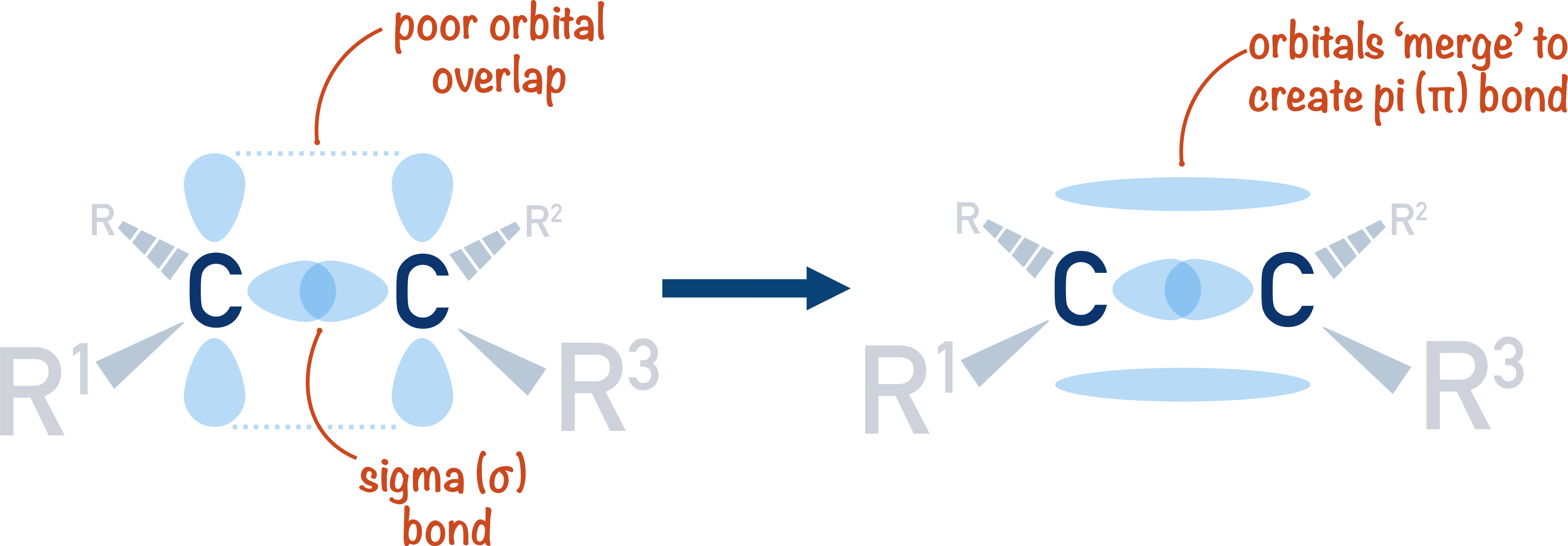
Can P Orbitals Form Sigma Bonds
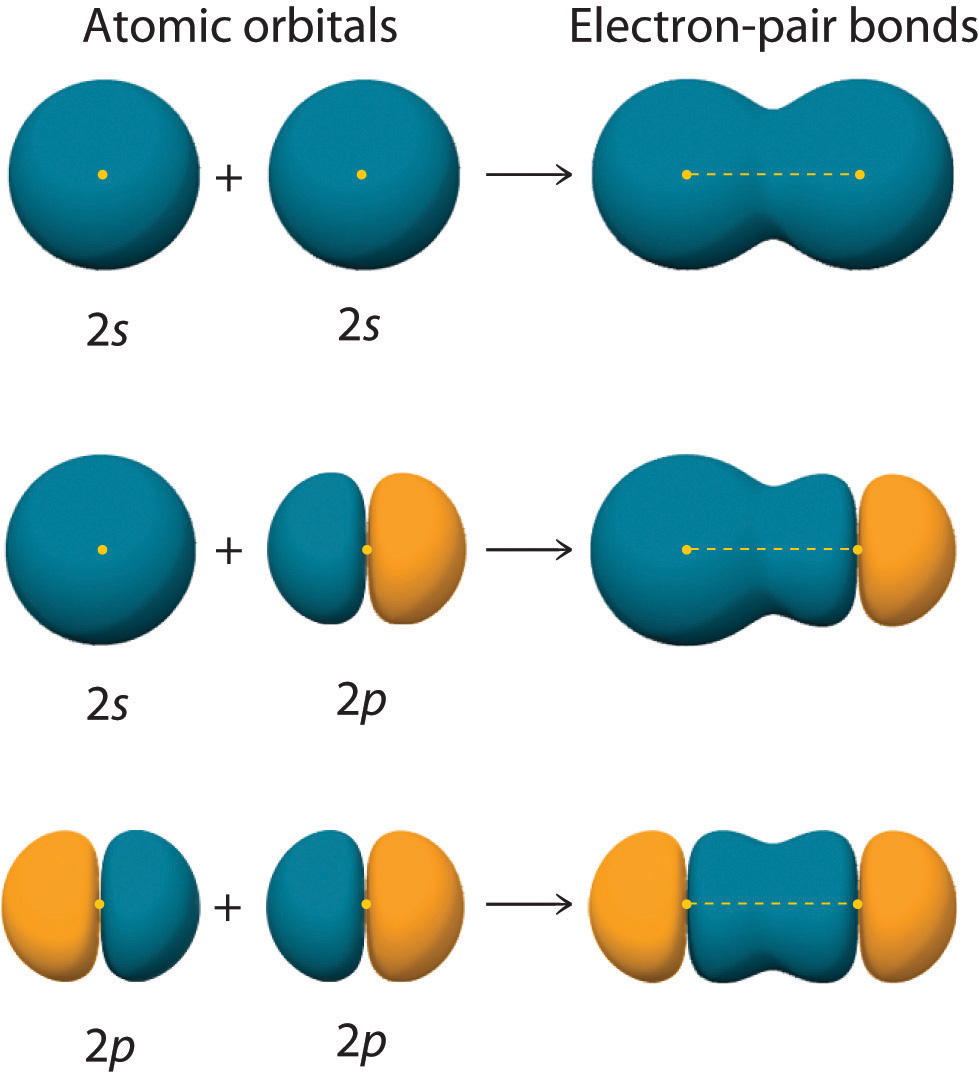
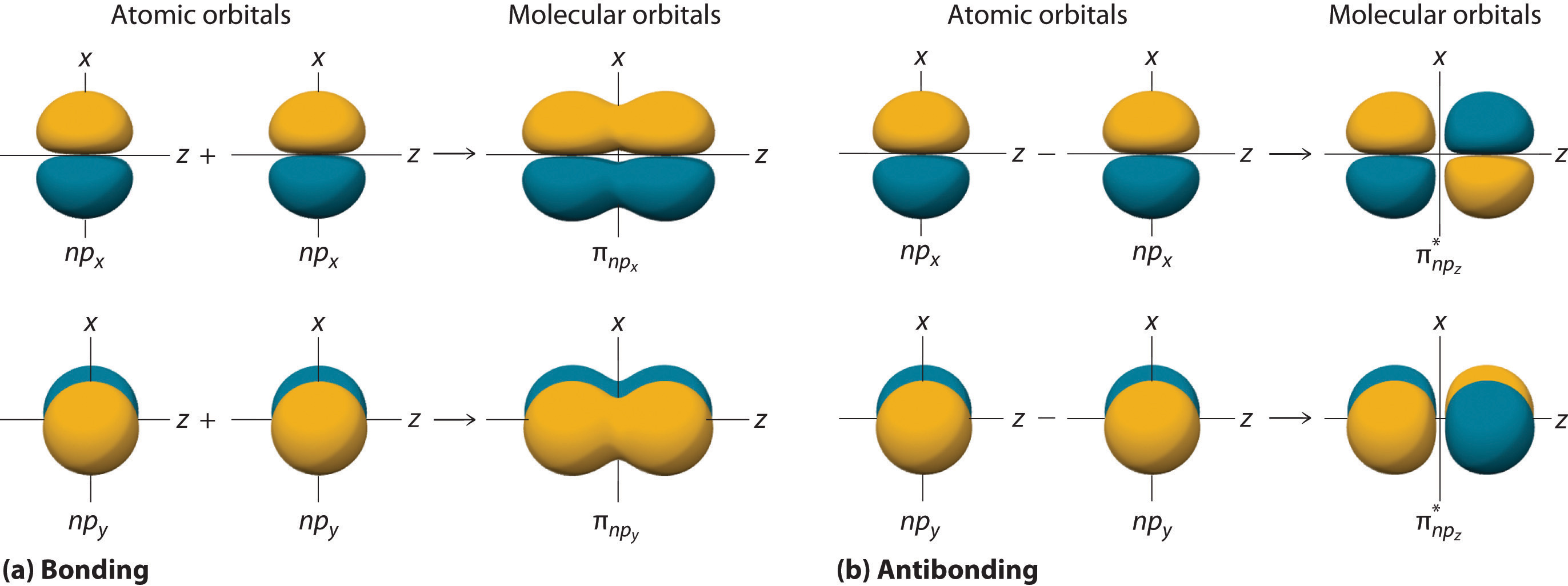
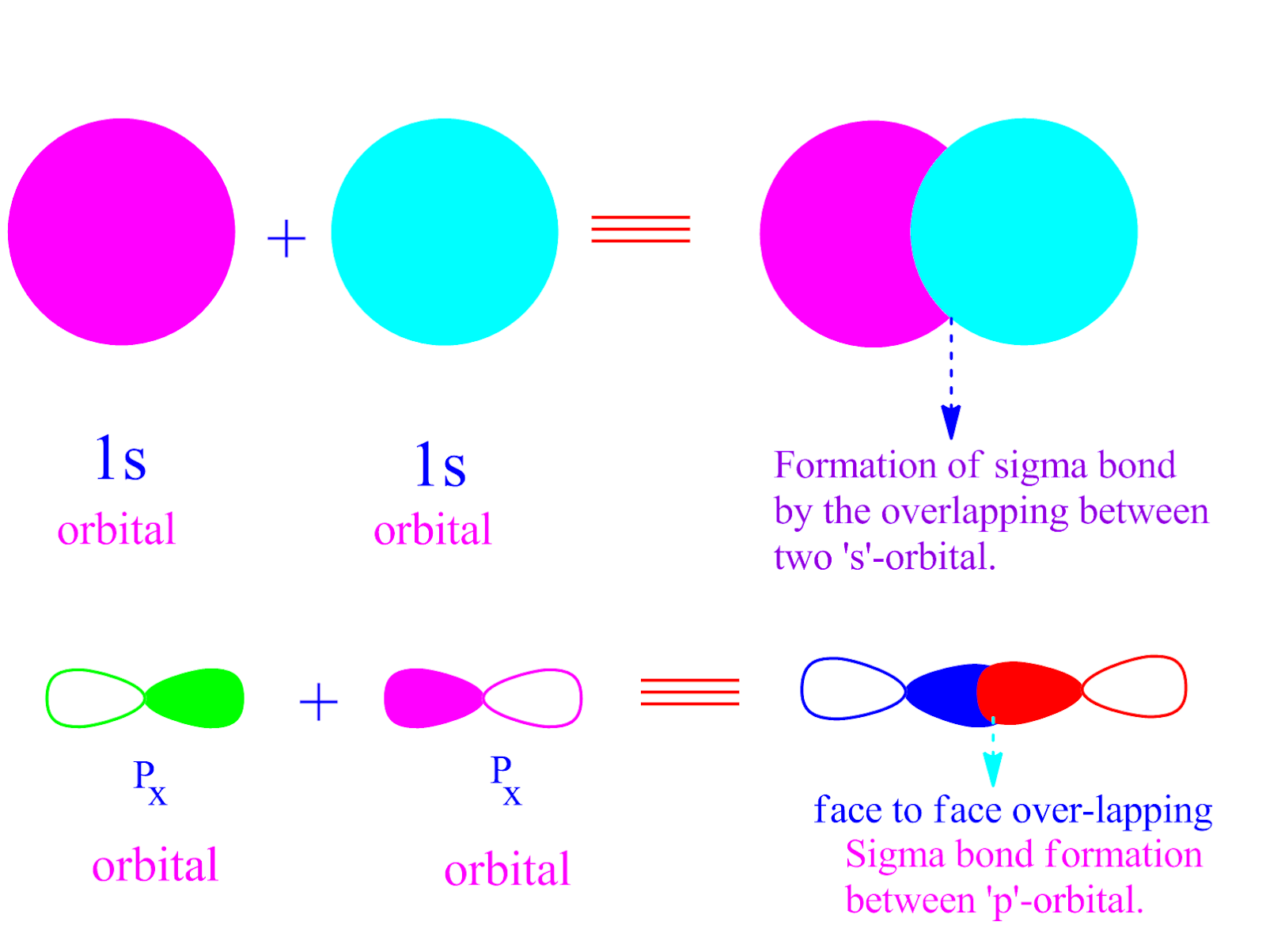
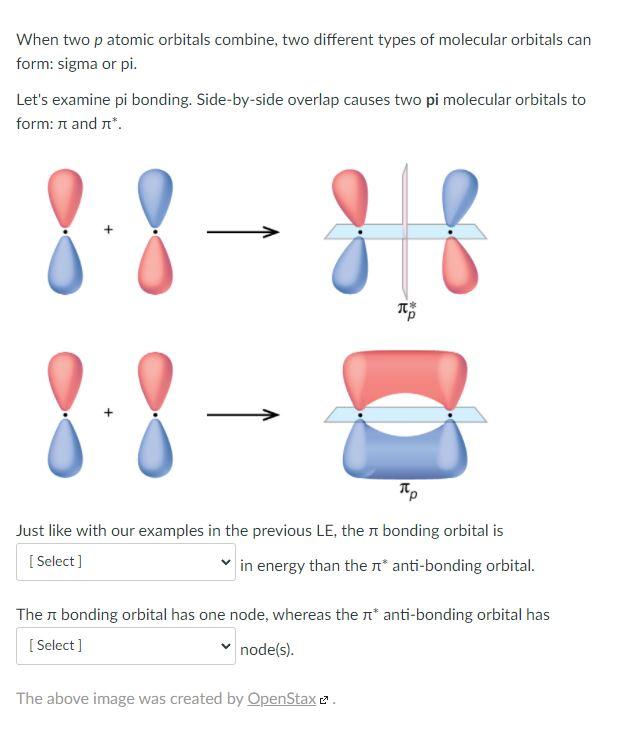
Can P Orbitals Form Sigma Bonds - A sigma bond can also be formed by the overlap of two p orbitals. A pi (π) orbital is one that has one node. This type of overlap allows the electron density to be concentrated along. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the.
Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node. A sigma bond can also be formed by the overlap of two p orbitals. This type of overlap allows the electron density to be concentrated along.
Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node. This type of overlap allows the electron density to be concentrated along. A sigma bond can also be formed by the overlap of two p orbitals.
2p Orbitals
This type of overlap allows the electron density to be concentrated along. A pi (π) orbital is one that has one node. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A sigma bond can also be formed by the overlap of two p orbitals. Any two orbitals (s, p, or d) that are.
9.3 Molecular Orbital Theory Chemistry LibreTexts
The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. This type of overlap allows the electron density to be concentrated along. A sigma bond can also be formed by the overlap of two p orbitals. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond..
8 Drawing Molecular Orbital Diagrams — Flux Science
The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A sigma bond can also be formed by the overlap of two p orbitals. A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. This type.
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. A pi (π) orbital is one that has one node. A sigma bond can also be formed by the overlap of two p orbitals. This type of overlap allows the electron density to be concentrated along. The covalent bond in molecular.
A π bond is formed by the overlap of
A sigma bond can also be formed by the overlap of two p orbitals. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. This type of overlap allows the electron density to be concentrated along..
Sigma和π键聪明的数学和科学Wiki
The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node. This type of overlap allows the electron density to be concentrated along. A sigma bond can also be formed by the overlap of two p orbitals. Any two orbitals (s, p, or d) that are.
Solved When two p atomic orbitals combine, two different
A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. This type.
Pi Bond And Sigma Bond How to count sigma and pi bonds Quora 3
This type of overlap allows the electron density to be concentrated along. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node. A sigma bond can also be formed by the overlap of two p orbitals. Any two orbitals (s, p, or d) that are.
sigma /r/okbuddyretard OkBuddyRetard Know Your Meme
This type of overlap allows the electron density to be concentrated along. A sigma bond can also be formed by the overlap of two p orbitals. A pi (π) orbital is one that has one node. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. Any two orbitals (s, p, or d) that are.
Alkenes (ALevel) ChemistryStudent
The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. A sigma bond can also be formed by the overlap of two p orbitals. This type.
This Type Of Overlap Allows The Electron Density To Be Concentrated Along.
Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node.