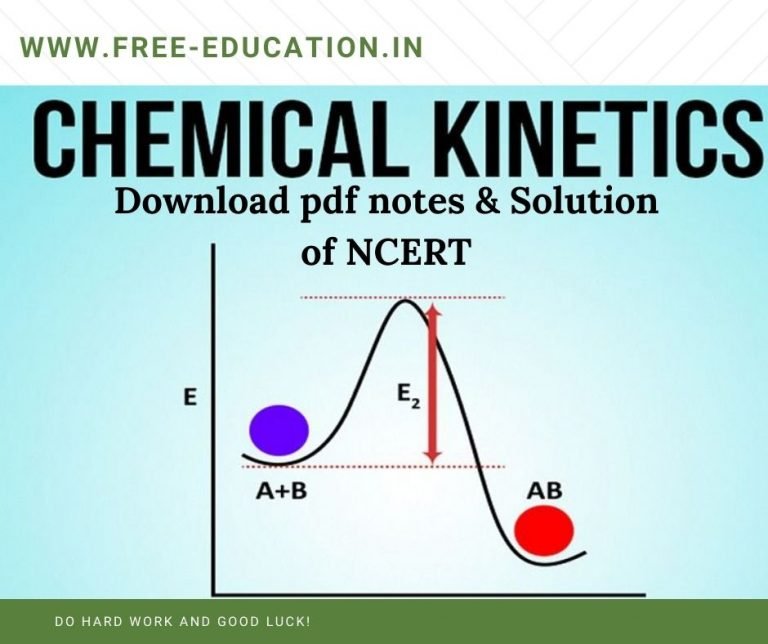
Chemical Kinetics Khan Academy
Chemical Kinetics Khan Academy - The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: Level up on all the skills in this unit and collect up to 700 mastery points! A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs.
The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. Level up on all the skills in this unit and collect up to 700 mastery points!
The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. Level up on all the skills in this unit and collect up to 700 mastery points!
Chemical 1 Chemical Chemistry basics, Chemistry
Check out the next lesson and practice what you’re learning: A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Level up on all the skills in this unit and collect.
SOLUTION Class 12 Chemistry Chapter 4 Chemical
Level up on all the skills in this unit and collect up to 700 mastery points! Check out the next lesson and practice what you’re learning: The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. A reaction mechanism is the sequence of elementary steps by.
(PDF) Chemical Ars Chemia · Chemical Chemical
The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. Level up on all the skills in this unit and collect up to 700 mastery points! Check out the next lesson.
CBSE Class 12th Chemistry Chemical Notes Wisdom TechSavvy
Level up on all the skills in this unit and collect up to 700 mastery points! The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: A reaction mechanism is the sequence of elementary steps by.
CHEMICAL Part5 By CHETNA SINGH, SCIENCE ACADEMY RENWAL YouTube
Level up on all the skills in this unit and collect up to 700 mastery points! Check out the next lesson and practice what you’re learning: The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. A reaction mechanism is the sequence of elementary steps by.
james e.house 2nd edition
Level up on all the skills in this unit and collect up to 700 mastery points! Check out the next lesson and practice what you’re learning: A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or.
CHEMICAL THEORY CHEMISTRY BY MUKESH SHARMA
Level up on all the skills in this unit and collect up to 700 mastery points! Check out the next lesson and practice what you’re learning: A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or.
Chemical 1 C B I T CHEMICAL Chemical
Check out the next lesson and practice what you’re learning: A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. Level up on all the skills in this unit and collect up to 700 mastery points! The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or.
Secondorder reactions AP Chemistry Khan Academy YouTube
A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. Check out the next lesson and practice what you’re learning: The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Level up on all the skills in this unit and collect.
(PPT) Chemical Students DOKUMEN.TIPS
A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. Level up on all the skills in this unit and collect up to 700 mastery points! Check out the next lesson and practice what you’re learning: The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or.
Check Out The Next Lesson And Practice What You’re Learning:
The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Level up on all the skills in this unit and collect up to 700 mastery points! A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs.