Clinical Trial Application Form
Clinical Trial Application Form - Learn how to be sure you are using the right. Describe past experimental and/or clinical findings leading to. There are two types of clinical studies: Does your human subjects study meet the nih definition of a clinical trial? For other fda forms, visit the fda forms page. Provide an introduction and background information. This page provides links to commonly used clinical trial forms relevant to clinical trials. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Interventional studies (also called clinical trials) and observational studies.
There are two types of clinical studies: For other fda forms, visit the fda forms page. Interventional studies (also called clinical trials) and observational studies. Describe past experimental and/or clinical findings leading to. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Does your human subjects study meet the nih definition of a clinical trial? Provide an introduction and background information. This page provides links to commonly used clinical trial forms relevant to clinical trials. Learn how to be sure you are using the right.
For other fda forms, visit the fda forms page. Provide an introduction and background information. There are two types of clinical studies: Describe past experimental and/or clinical findings leading to. Interventional studies (also called clinical trials) and observational studies. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Learn how to be sure you are using the right. Does your human subjects study meet the nih definition of a clinical trial? This page provides links to commonly used clinical trial forms relevant to clinical trials.
Clinical Trial Registration and Application Form Clinical Research
Learn how to be sure you are using the right. There are two types of clinical studies: Interventional studies (also called clinical trials) and observational studies. Provide an introduction and background information. Does your human subjects study meet the nih definition of a clinical trial?
UK MHRA Guidance Clinical Trials for Medicines Apply for
The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms relevant to clinical trials. Interventional studies (also called clinical trials) and observational studies. Provide an introduction and background information.
Clinical Trial Application Form Fill Online, Printable, Fillable
Does your human subjects study meet the nih definition of a clinical trial? This page provides links to commonly used clinical trial forms relevant to clinical trials. Learn how to be sure you are using the right. For other fda forms, visit the fda forms page. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and.
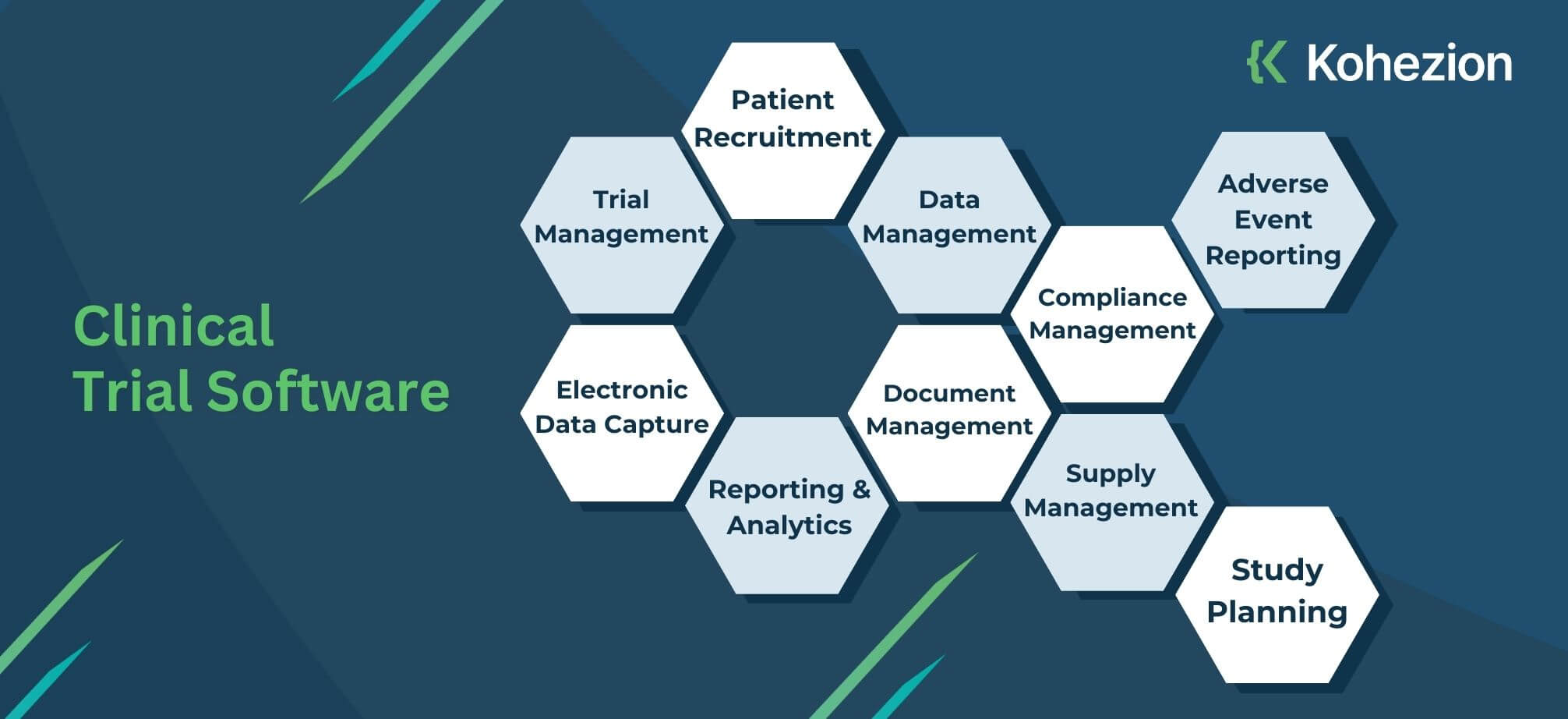
Custom Clinical Trial Software
The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Does your human subjects study meet the nih definition of a clinical trial? Interventional studies (also called clinical trials) and observational studies. Learn how to be sure you are using the right. There are two types of clinical studies:
C IRB Clinical Trial Application Form
This page provides links to commonly used clinical trial forms relevant to clinical trials. Describe past experimental and/or clinical findings leading to. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. There are two types of clinical studies: Learn how to be sure you are using the right.
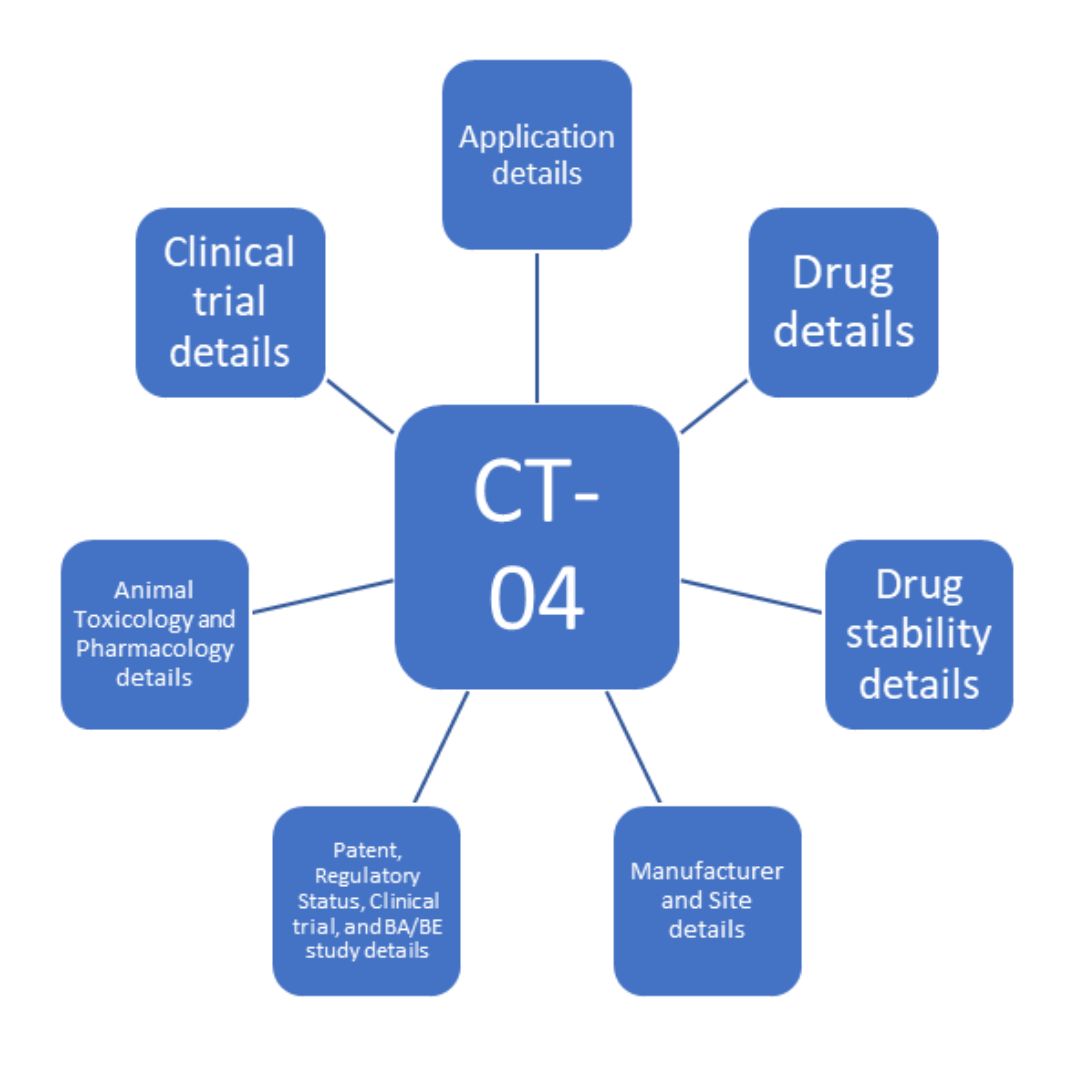
Clinical Trial Application and Import Requirements in India with
Interventional studies (also called clinical trials) and observational studies. Does your human subjects study meet the nih definition of a clinical trial? Provide an introduction and background information. For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms relevant to clinical trials.
Fillable Online Application Form for a New Clinical Trial.doc Fax Email
This page provides links to commonly used clinical trial forms relevant to clinical trials. Describe past experimental and/or clinical findings leading to. Learn how to be sure you are using the right. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Does your human subjects study meet the nih definition of.
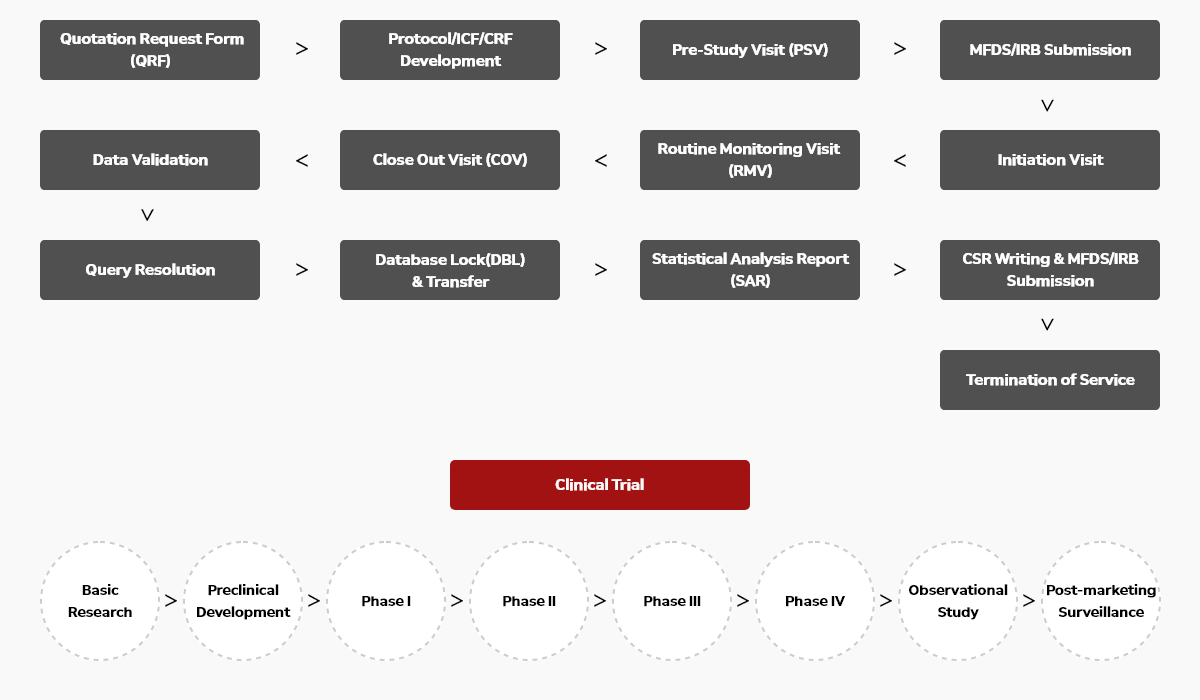
Clinical Trial Process intoinworld
Interventional studies (also called clinical trials) and observational studies. This page provides links to commonly used clinical trial forms relevant to clinical trials. For other fda forms, visit the fda forms page. Provide an introduction and background information. There are two types of clinical studies:
Clinical Trial Approval Process In Canada Credevo Articles
Provide an introduction and background information. Describe past experimental and/or clinical findings leading to. This page provides links to commonly used clinical trial forms relevant to clinical trials. There are two types of clinical studies: Does your human subjects study meet the nih definition of a clinical trial?
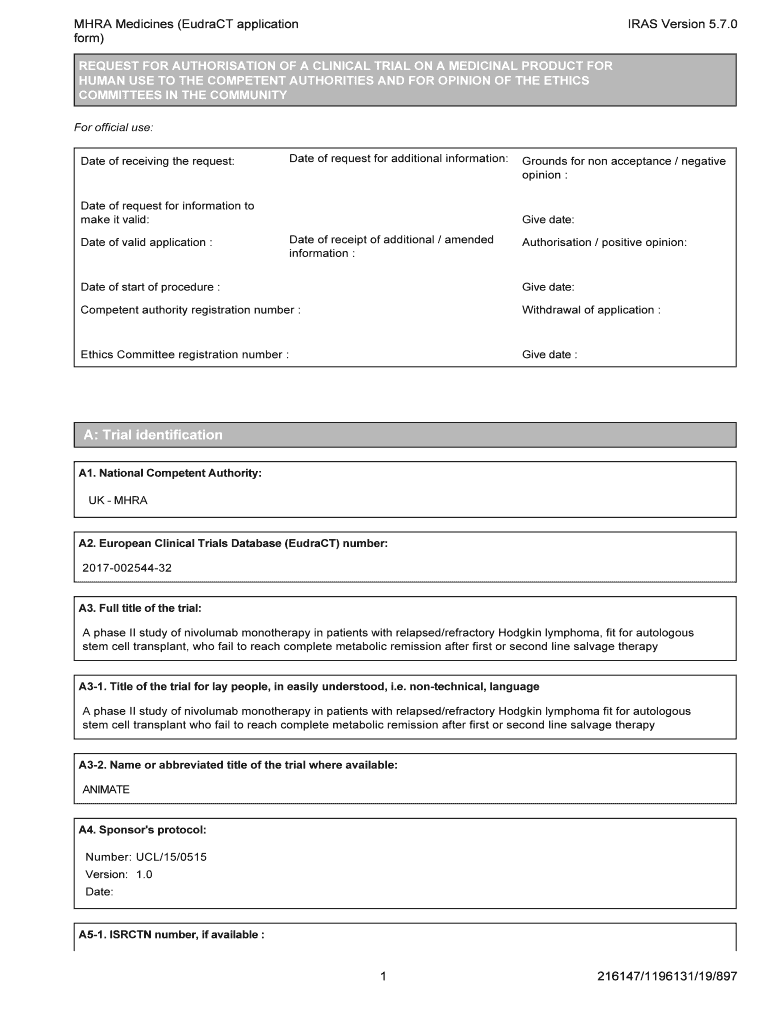
Fillable Online Clinical trial Application Form Europa Fax Email
This page provides links to commonly used clinical trial forms relevant to clinical trials. Interventional studies (also called clinical trials) and observational studies. Does your human subjects study meet the nih definition of a clinical trial? Describe past experimental and/or clinical findings leading to. Provide an introduction and background information.
Interventional Studies (Also Called Clinical Trials) And Observational Studies.
Does your human subjects study meet the nih definition of a clinical trial? Describe past experimental and/or clinical findings leading to. Provide an introduction and background information. For other fda forms, visit the fda forms page.
The Phs Human Subjects And Clinical Trial Form Consolidates Human Subjects, Inclusion Enrollment, And Clinical Trial Information Into.
There are two types of clinical studies: This page provides links to commonly used clinical trial forms relevant to clinical trials. Learn how to be sure you are using the right.