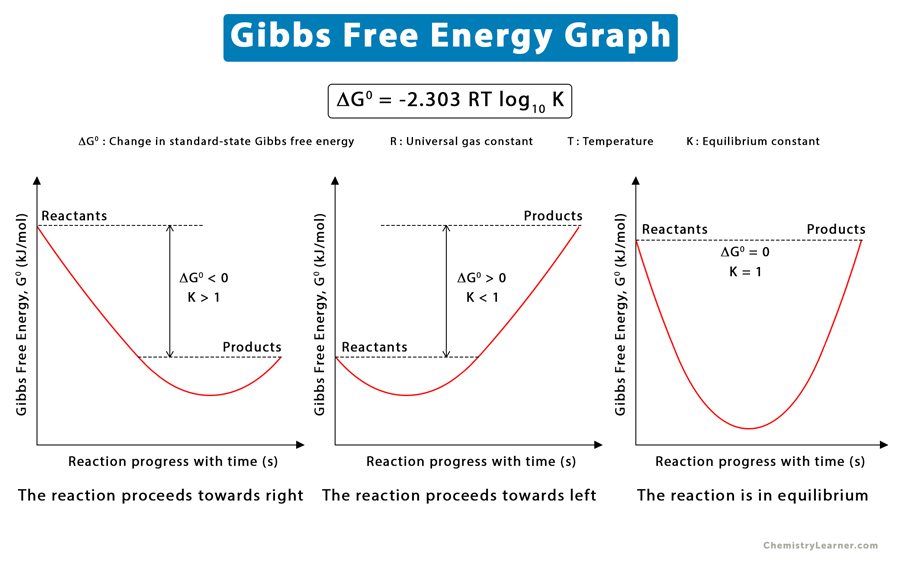
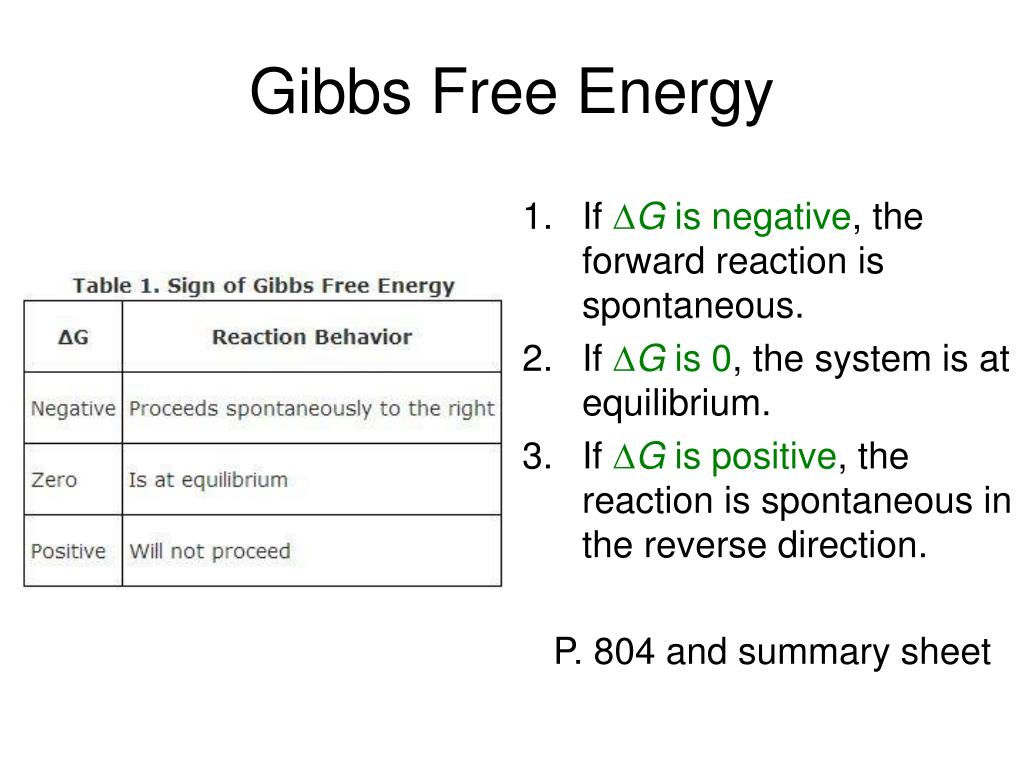
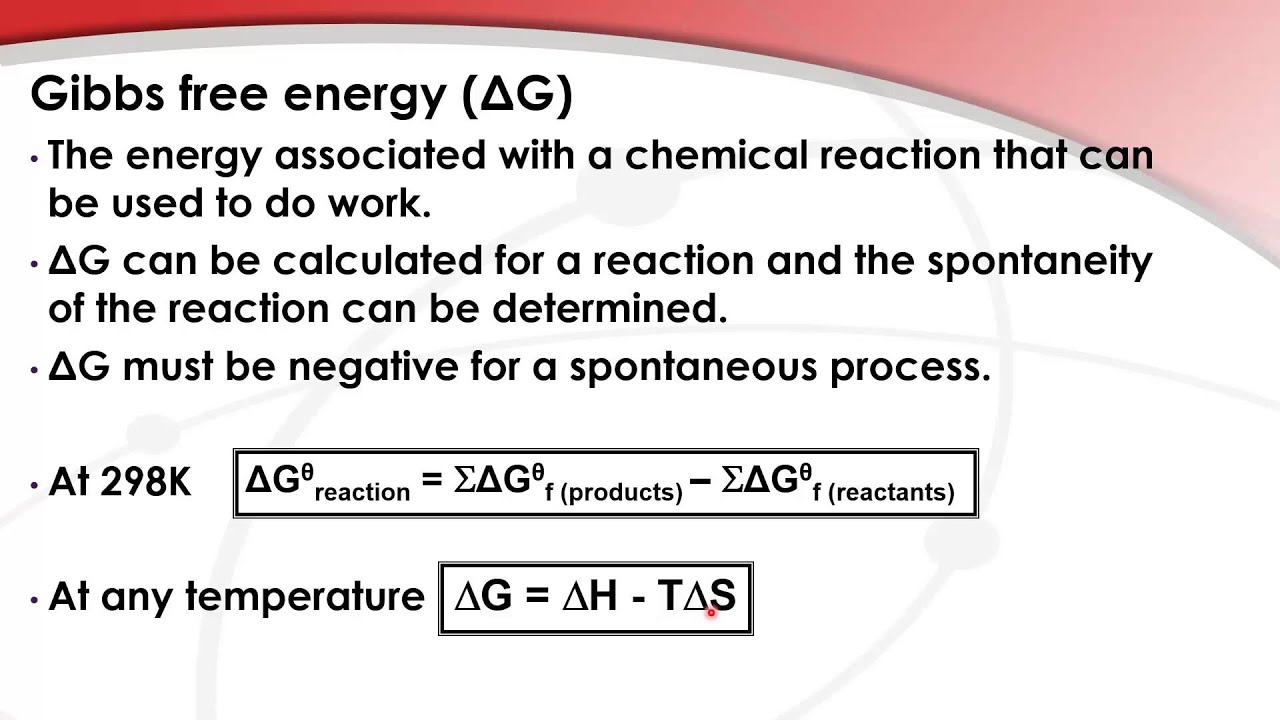
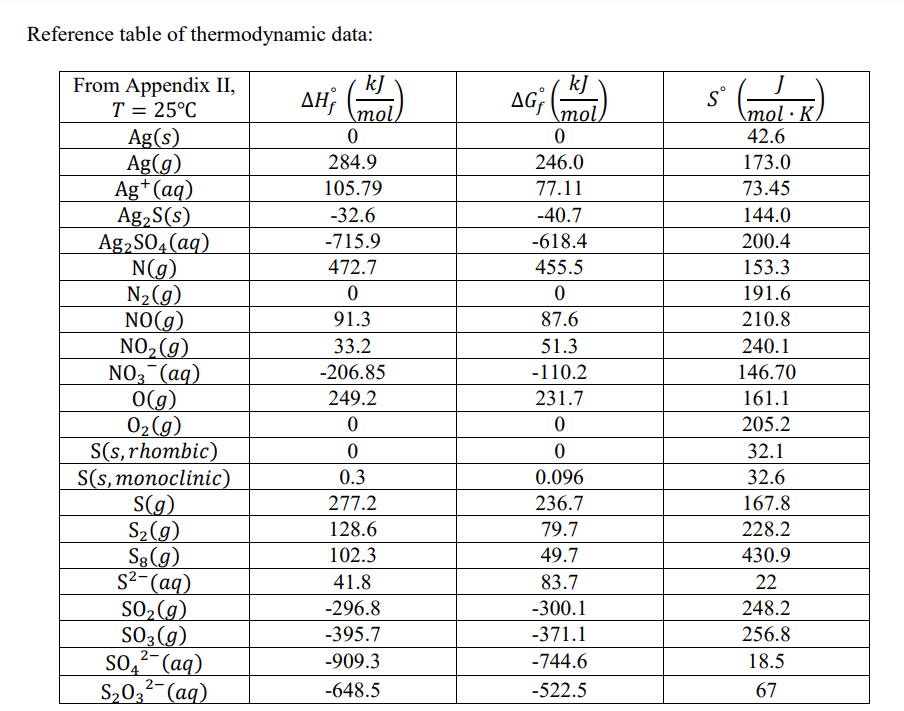
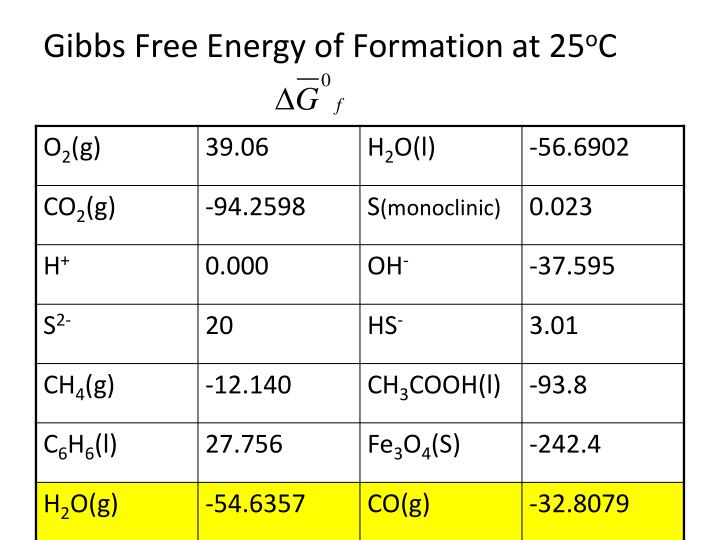
Gibbs Free Energy Of Formation
Gibbs Free Energy Of Formation - Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Gibbs free energy, denoted g, combines enthalpy and entropy into a single value. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. The change in free energy, δg, is equal. Find out how δg determines.
Find out how δg determines. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. The change in free energy, δg, is equal. Gibbs free energy, denoted g, combines enthalpy and entropy into a single value.
Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. Gibbs free energy, denoted g, combines enthalpy and entropy into a single value. The change in free energy, δg, is equal. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Find out how δg determines.
Gibbs Free Energy Definition, Equation, Unit, and Example
Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. The change in free energy, δg, is equal. Gibbs free energy, denoted g, combines enthalpy and entropy into a single value. Find out how δg determines. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change.
PPT Gibbs Free Energy PowerPoint Presentation, free download ID2198185
The change in free energy, δg, is equal. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Find out how δg determines. Learn how.
Gibbs’ Free Energy Function Overall Science
Gibbs free energy, denoted g, combines enthalpy and entropy into a single value. Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. The change in free energy, δg, is equal. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that.
Solved 6A. Using reference Gibbs free energies of formation
129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Gibbs free energy, denoted g, combines enthalpy and entropy into a single value. Find out.
Schematic illustration of microstructures and Gibbs free energy curves
Find out how δg determines. The change in free energy, δg, is equal. Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Definition and explanation of the terms.
PPT Useful Equations The Clapeyron Equation PowerPoint Presentation
Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Find out how δg determines. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Gibbs free energy, denoted g, combines enthalpy and entropy into.
Standard Gibbs's free energy of the nitride formation reaction
Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The change in free energy, δg, is equal. Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. Find out how δg determines. 129 rows the standard gibbs free energy of formation.
Gibbs free energy for formation of relating oxides and oxygen potential
Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. The change in free energy, δg, is equal. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 129 rows the standard gibbs free energy of formation (gf °) of a compound.
Comparison of Gibbs free energy change, ΔG, of the formation of Al
Find out how δg determines. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Learn how to calculate gibbs free energy change (δg) for.
Gibbs free energy of formation for various oxides and sulfides
The change in free energy, δg, is equal. Find out how δg determines. Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Gibbs free energy, denoted g, combines.
The Change In Free Energy, Δg, Is Equal.
Gibbs free energy, denoted g, combines enthalpy and entropy into a single value. 129 rows the standard gibbs free energy of formation (gf °) of a compound is the change of gibbs free energy that accompanies the. Find out how δg determines. Learn how to calculate gibbs free energy change (δg) for chemical reactions using enthalpy and entropy changes.