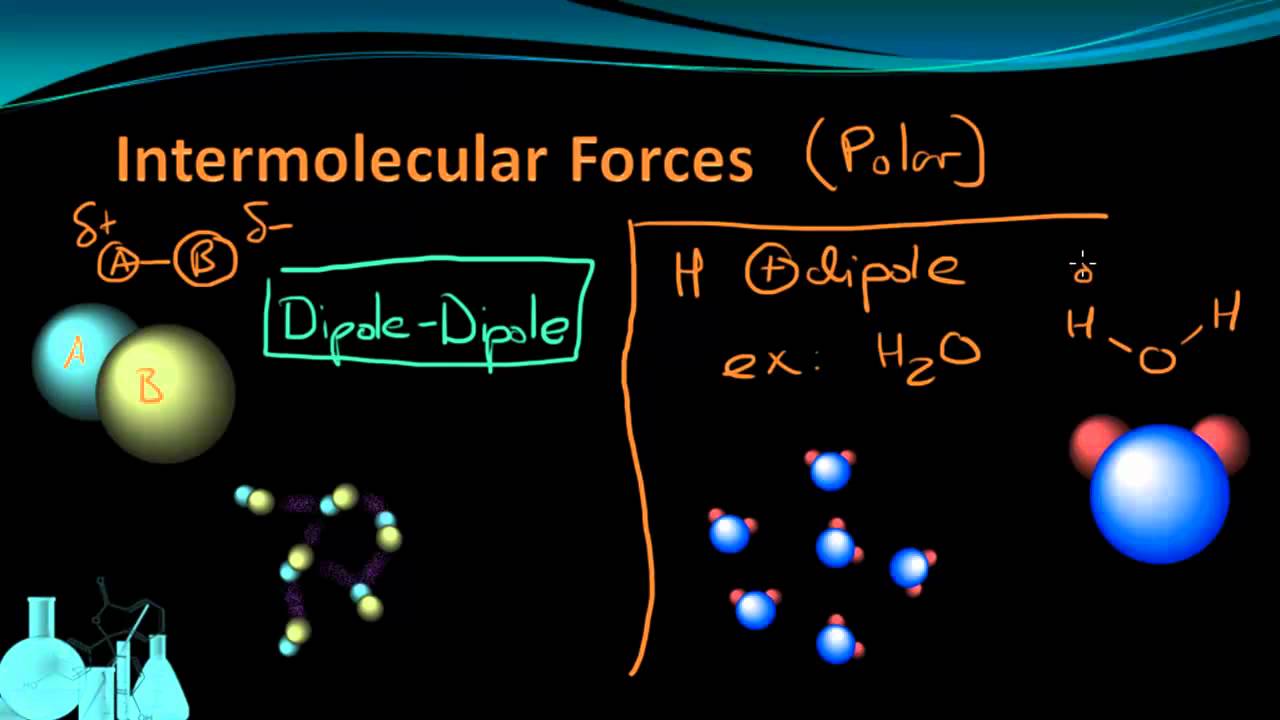
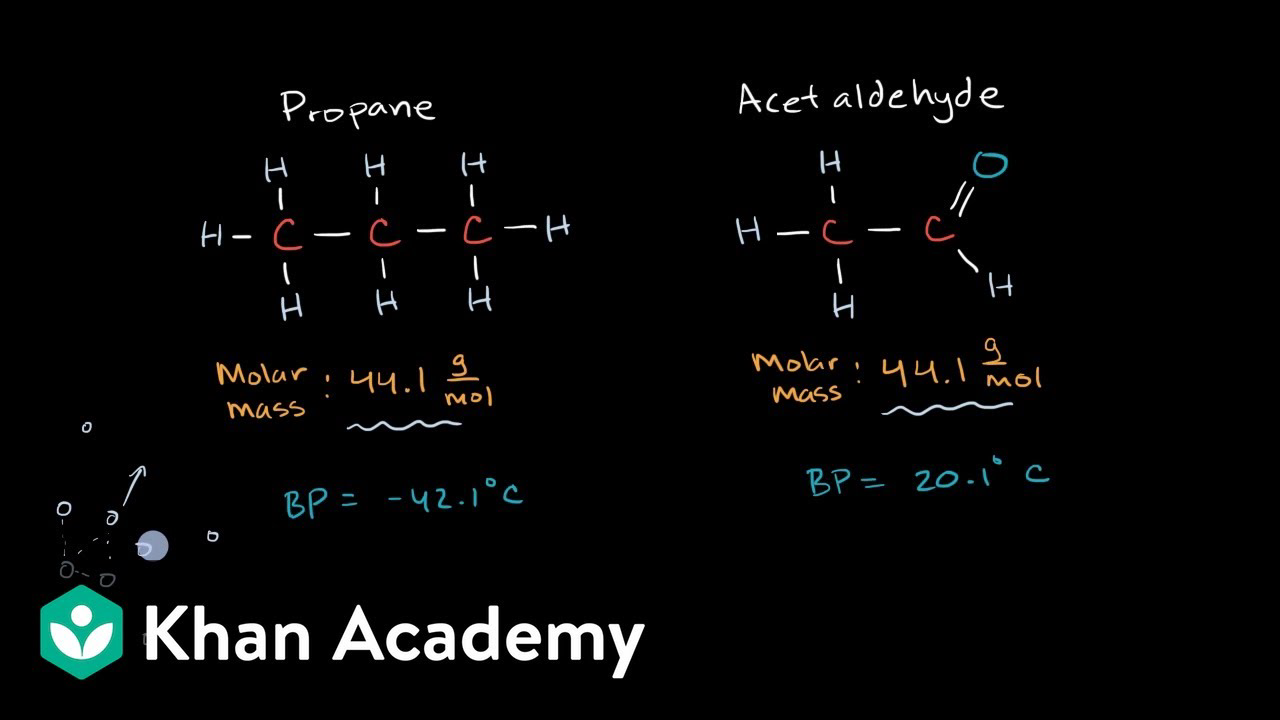
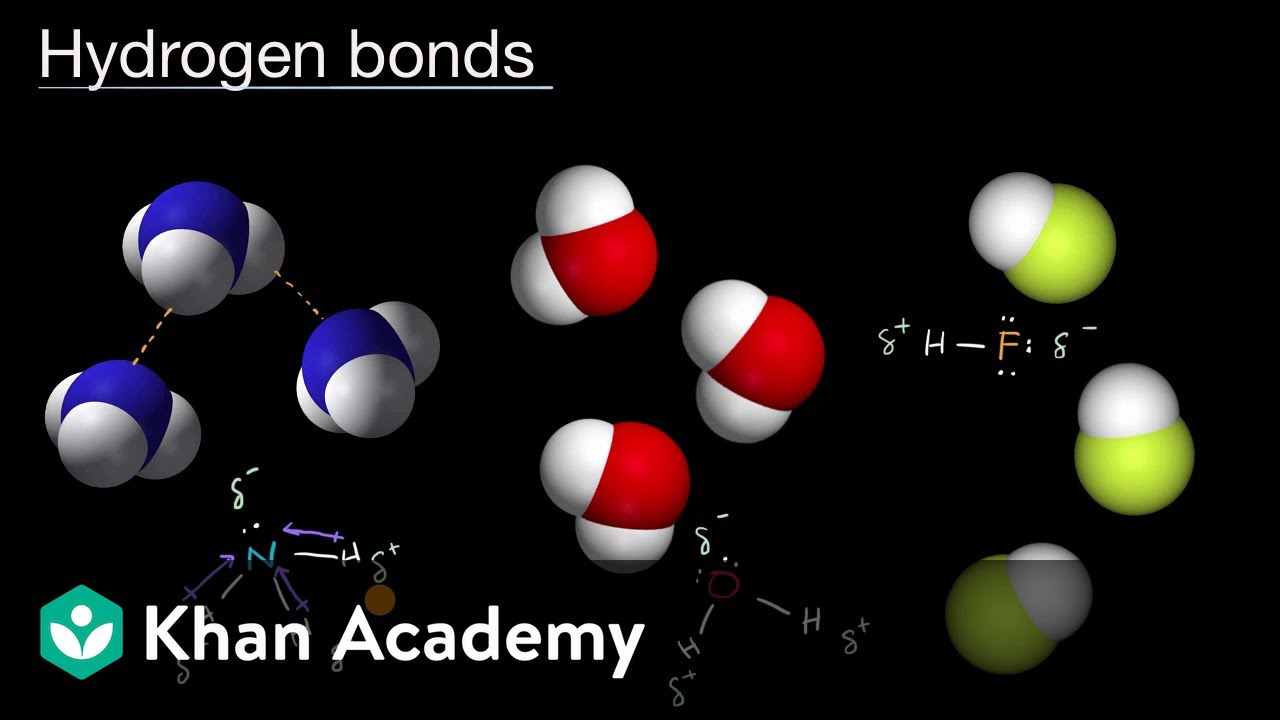
Khan Academy Intermolecular Forces
Khan Academy Intermolecular Forces - The stronger these forces, the lower. Up to 2nd grade (khan kids) 2nd grade; We're a nonprofit that relies on support from people like you. 6th grade reading and vocab. Substances with similar polarities tend to be soluble in one another (like dissolves like). We'll get right to the point: A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. 7th grade reading and vocab. Nonpolar substances are generally more. We're asking you to help support khan academy.
We're asking you to help support khan academy. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Nonpolar substances are generally more. 6th grade reading and vocab. We'll get right to the point: We're a nonprofit that relies on support from people like you. Substances with similar polarities tend to be soluble in one another (like dissolves like). The stronger these forces, the lower. Up to 2nd grade (khan kids) 2nd grade; 7th grade reading and vocab.
6th grade reading and vocab. 7th grade reading and vocab. The stronger these forces, the lower. Substances with similar polarities tend to be soluble in one another (like dissolves like). Nonpolar substances are generally more. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. We're asking you to help support khan academy. We're a nonprofit that relies on support from people like you. We'll get right to the point: Up to 2nd grade (khan kids) 2nd grade;
Van der Waals forces States of matter and intermolecular forces
Nonpolar substances are generally more. Substances with similar polarities tend to be soluble in one another (like dissolves like). We're asking you to help support khan academy. The stronger these forces, the lower. We're a nonprofit that relies on support from people like you.
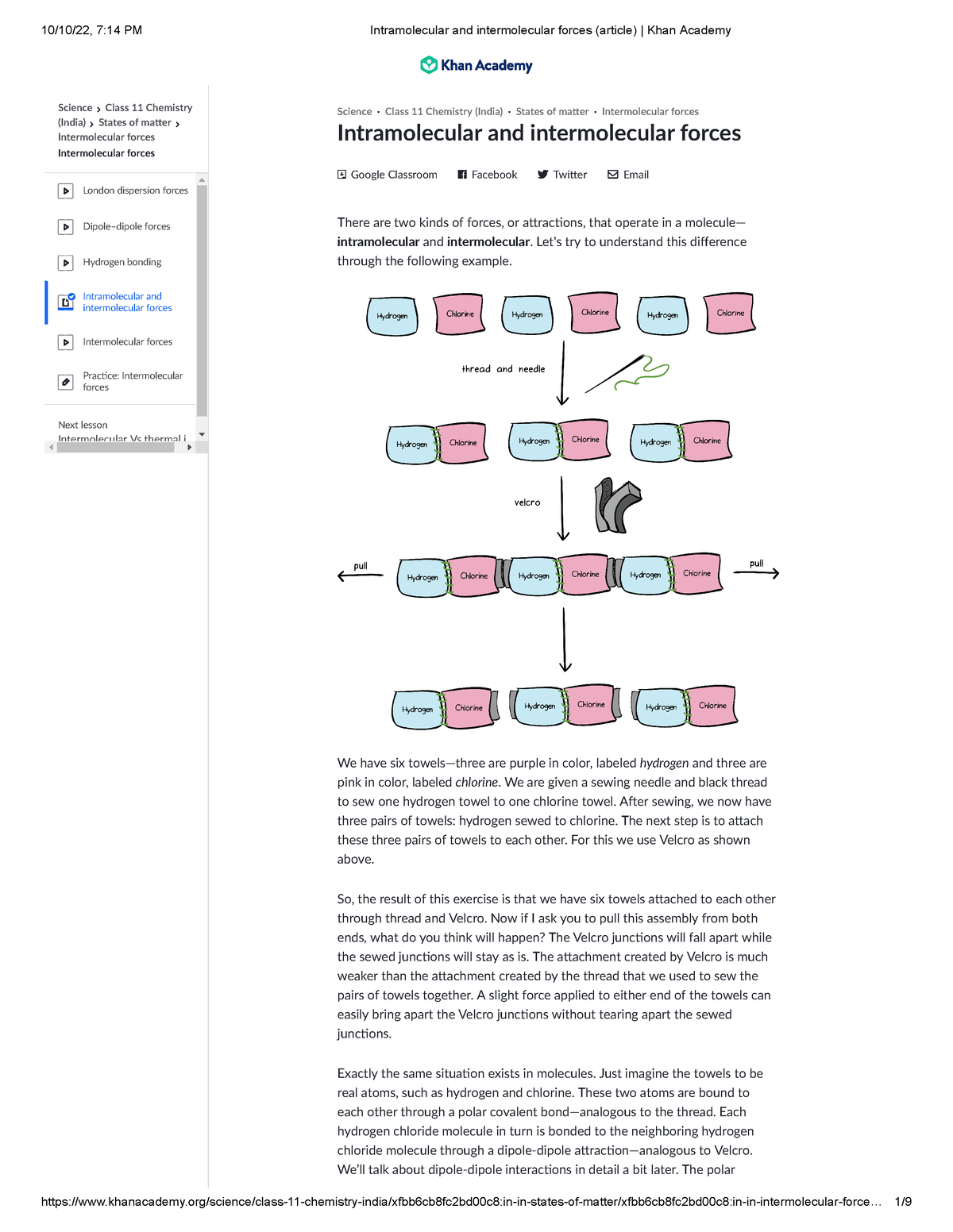
Intramolecular and intermolecular forces (article) Khan Academy
7th grade reading and vocab. 6th grade reading and vocab. We're asking you to help support khan academy. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Up to 2nd grade (khan kids) 2nd grade;
Intramolecular vs Intermolecular Forces YouTube
We'll get right to the point: 7th grade reading and vocab. Nonpolar substances are generally more. Substances with similar polarities tend to be soluble in one another (like dissolves like). We're asking you to help support khan academy.
[DIAGRAM] Ch3och3 Intermolecular Forces Diagram
A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. 6th grade reading and vocab. 7th grade reading and vocab. We're a nonprofit that relies on support from people like you. Substances with similar polarities tend to be soluble in one another (like dissolves like).
Intermolecular Forces Lessons Blendspace
7th grade reading and vocab. Substances with similar polarities tend to be soluble in one another (like dissolves like). We're asking you to help support khan academy. We'll get right to the point: Nonpolar substances are generally more.
Khan Academy Breakthrough Challenge Intermolecular Forces and
6th grade reading and vocab. Substances with similar polarities tend to be soluble in one another (like dissolves like). Up to 2nd grade (khan kids) 2nd grade; We're a nonprofit that relies on support from people like you. The stronger these forces, the lower.
Intramolecular and intermolecular forces (article) Khan Academy in
The stronger these forces, the lower. We're a nonprofit that relies on support from people like you. Up to 2nd grade (khan kids) 2nd grade; 6th grade reading and vocab. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules.
Intermolecular forces (apply) (practice) Khan Academy
Substances with similar polarities tend to be soluble in one another (like dissolves like). We're a nonprofit that relies on support from people like you. Up to 2nd grade (khan kids) 2nd grade; 6th grade reading and vocab. We're asking you to help support khan academy.
Khan Academy
We're a nonprofit that relies on support from people like you. Substances with similar polarities tend to be soluble in one another (like dissolves like). 7th grade reading and vocab. We're asking you to help support khan academy. We'll get right to the point:
Fillable Online Intramolecular and intermolecular forces (article)Khan
7th grade reading and vocab. Substances with similar polarities tend to be soluble in one another (like dissolves like). We'll get right to the point: We're a nonprofit that relies on support from people like you. The stronger these forces, the lower.
We're A Nonprofit That Relies On Support From People Like You.
The stronger these forces, the lower. Nonpolar substances are generally more. Substances with similar polarities tend to be soluble in one another (like dissolves like). We're asking you to help support khan academy.
We'll Get Right To The Point:
A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Up to 2nd grade (khan kids) 2nd grade; 6th grade reading and vocab. 7th grade reading and vocab.


![[DIAGRAM] Ch3och3 Intermolecular Forces Diagram](http://clipart-library.com/img1/1419819.png)