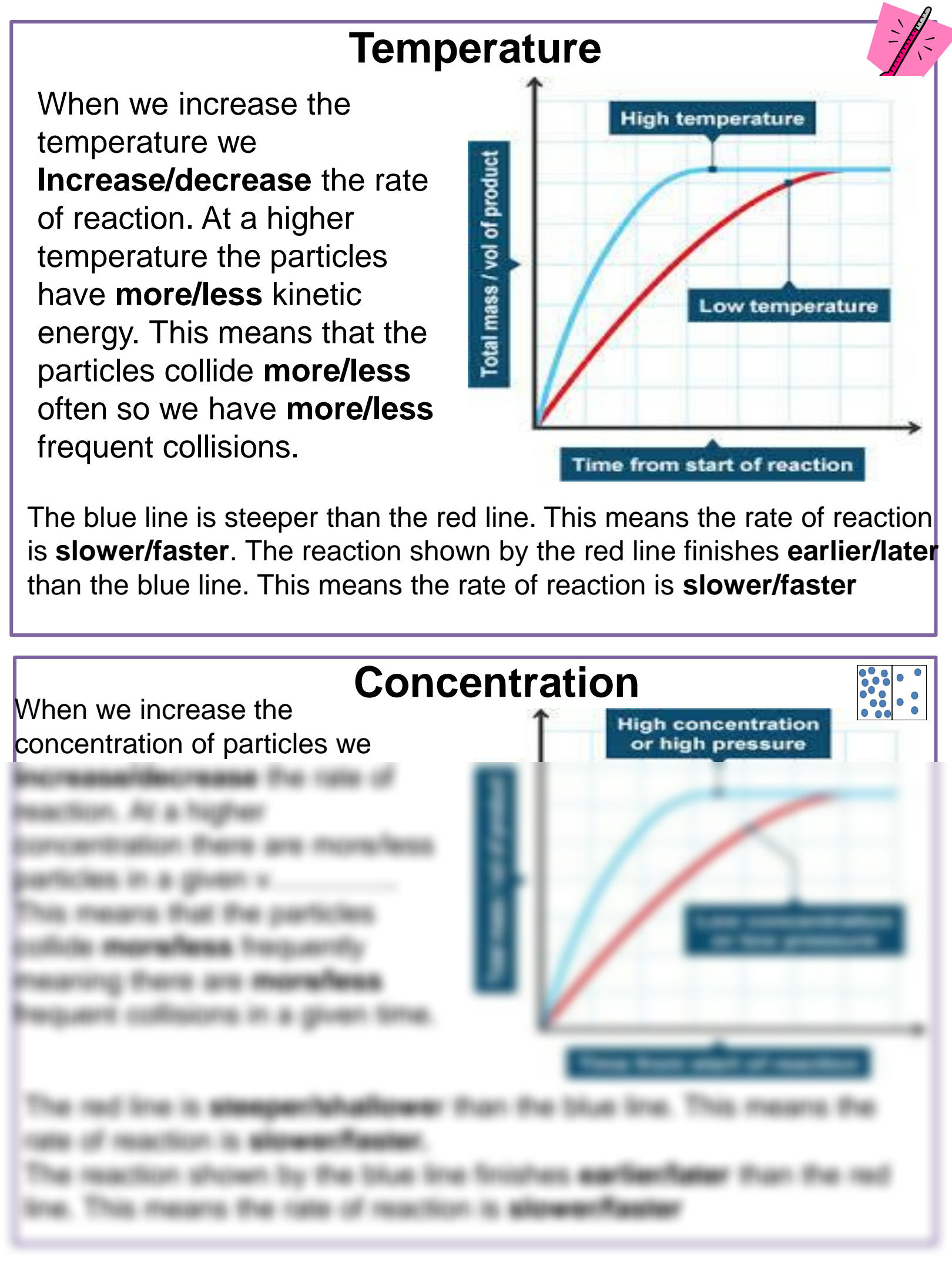
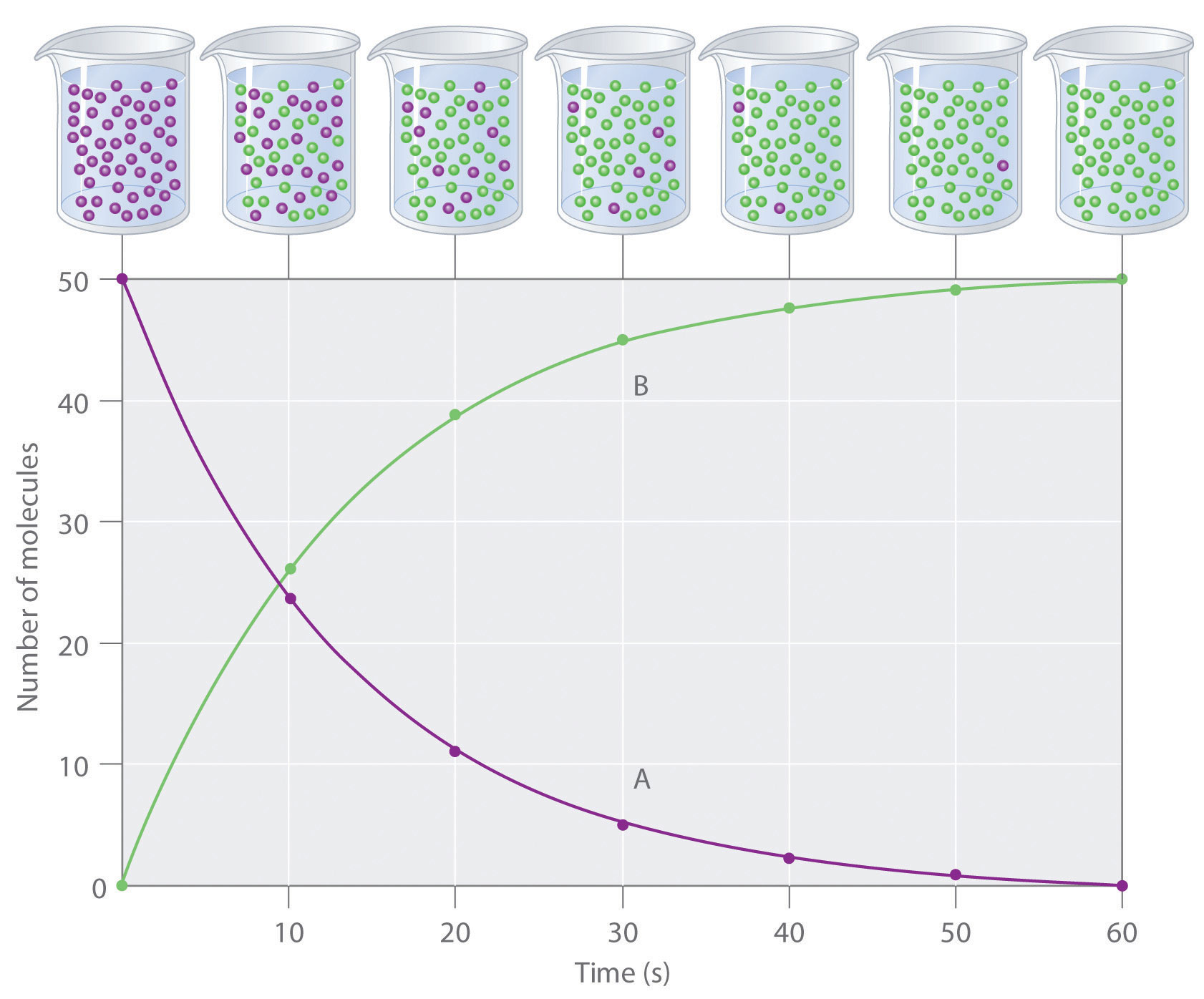
Reaction Rate Worksheet
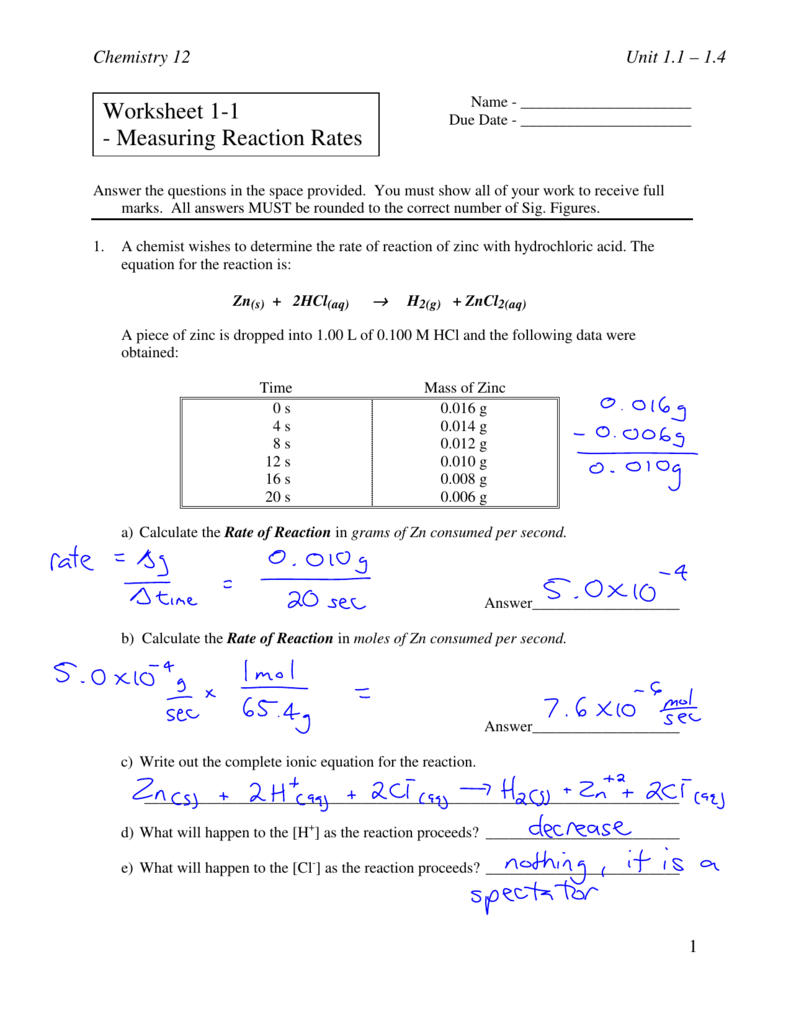
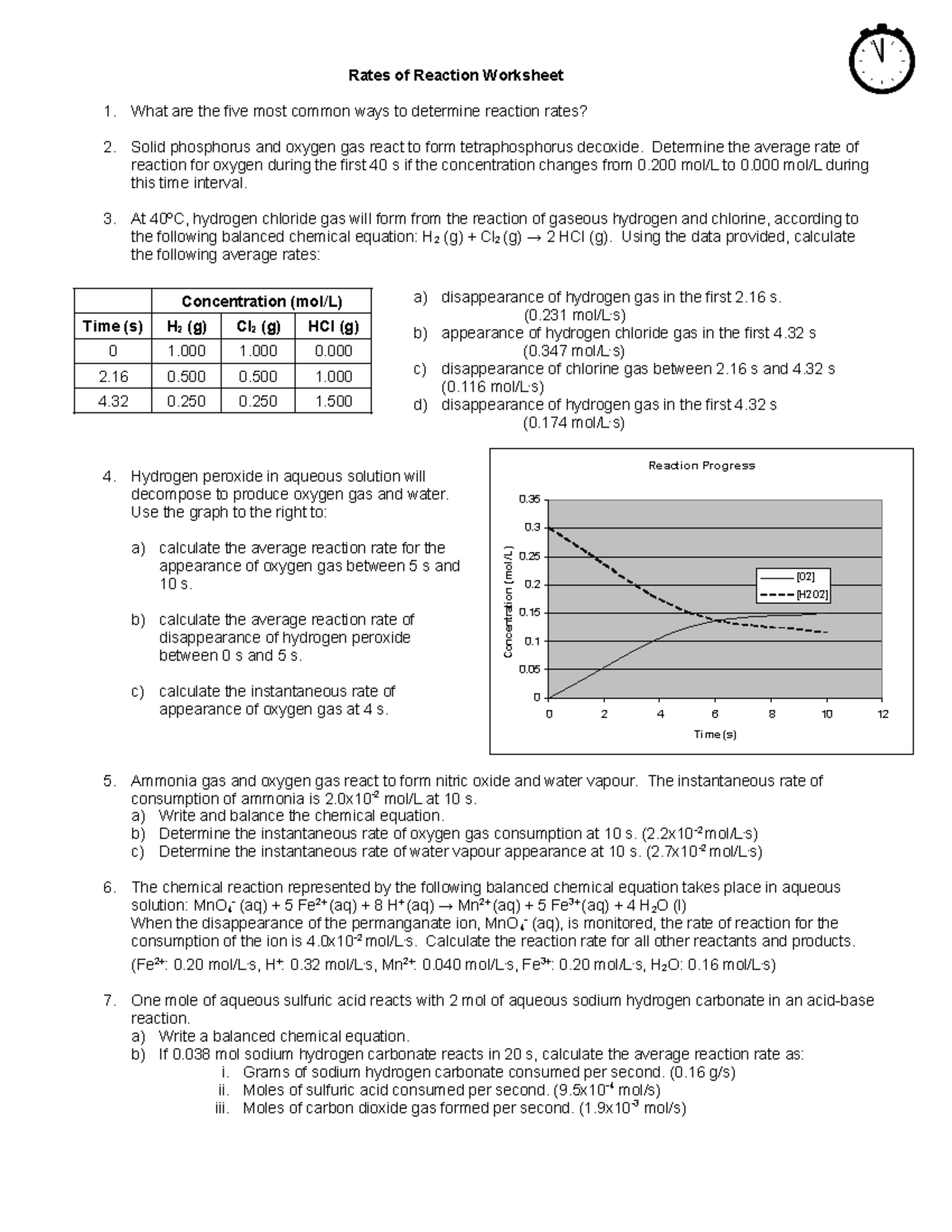
Reaction Rate Worksheet - A student investigated the rate of reaction between calcium carbonate (marble chips) and. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Calculate the mean rate of reaction from 0 to 15 seconds. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A study of reaction _____ is called chemical _____. Other reactions can take a very long time, like the. Give some examples of situations where we might want to decrease the rate of a particular reaction.
C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A study of reaction _____ is called chemical _____. A student investigated the rate of reaction between calcium carbonate (marble chips) and. Give some examples of situations where we might want to decrease the rate of a particular reaction. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine. Other reactions can take a very long time, like the. Calculate the mean rate of reaction from 0 to 15 seconds.
Give some examples of situations where we might want to decrease the rate of a particular reaction. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine. A study of reaction _____ is called chemical _____. Calculate the mean rate of reaction from 0 to 15 seconds. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. A student investigated the rate of reaction between calcium carbonate (marble chips) and. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Other reactions can take a very long time, like the.
Rate of Reaction Practical Home Learning Worksheet GCSE rocketsheets
Other reactions can take a very long time, like the. A study of reaction _____ is called chemical _____. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Rates of reaction some reactions occur very quickly, like the burning of.
Rates Of Reaction Worksheet —
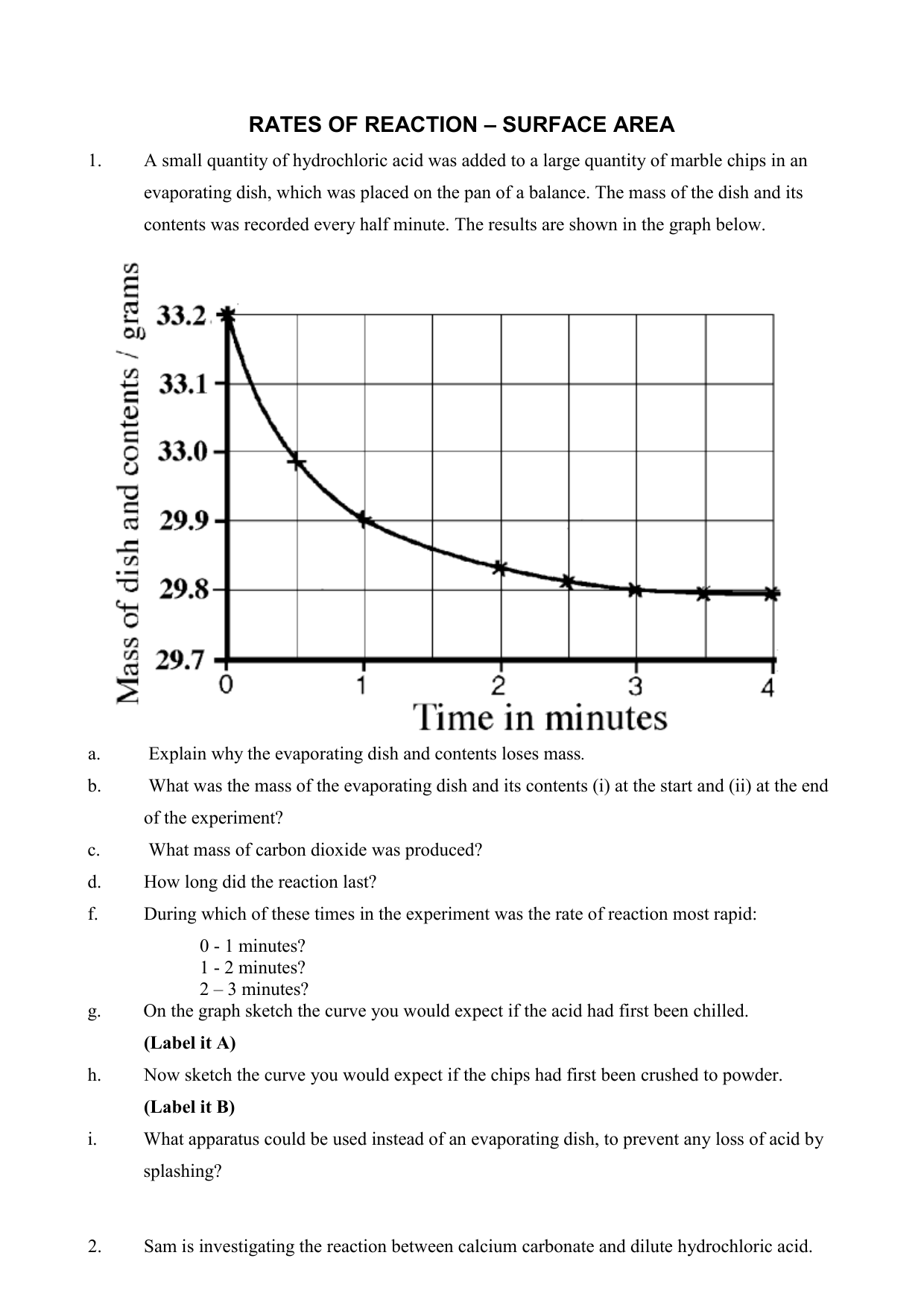
A student investigated the rate of reaction between calcium carbonate (marble chips) and. Other reactions can take a very long time, like the. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A study of reaction _____ is called chemical _____. Give some examples of situations.
Rate Of Reaction Chemistry Worksheet
Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Calculate the mean rate of reaction from 0.
reaction rates
A student investigated the rate of reaction between calcium carbonate (marble chips) and. Other reactions can take a very long time, like the. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid..
SOLUTION Factors affecting the rate of reaction worksheet Studypool
C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Calculate the mean rate of reaction from 0 to 15 seconds. Give some examples of situations where we might want to decrease the rate of a particular reaction. Rates of reaction some reactions occur very quickly, like.
12 u lesson 6 rates of reaction worksheet Rates of Reaction
A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Other reactions can take a very long time, like the. A study of reaction _____ is called chemical _____. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Reaction rate refers to.
RATES OF REACTION SURFACE AREA
Give some examples of situations where we might want to decrease the rate of a particular reaction. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A study of reaction _____ is called chemical _____. Rates of reaction some reactions occur very quickly, like the burning.
SOLUTION Factors affecting the rate of reaction worksheet Studypool
Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Calculate the mean rate of reaction from 0 to 15 seconds. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Give some examples of situations where we might want to decrease the rate of a particular reaction. Rates of reaction.
Rate of Reaction Grade 8 Worksheet PDF Catalysis Chemical
Other reactions can take a very long time, like the. Give some examples of situations where we might want to decrease the rate of a particular reaction. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A chemist wishes to determine the rate of reaction of.
Rate Of Reaction Worksheet Grade 8 Pdf
C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Rates of reaction some reactions occur very quickly,.
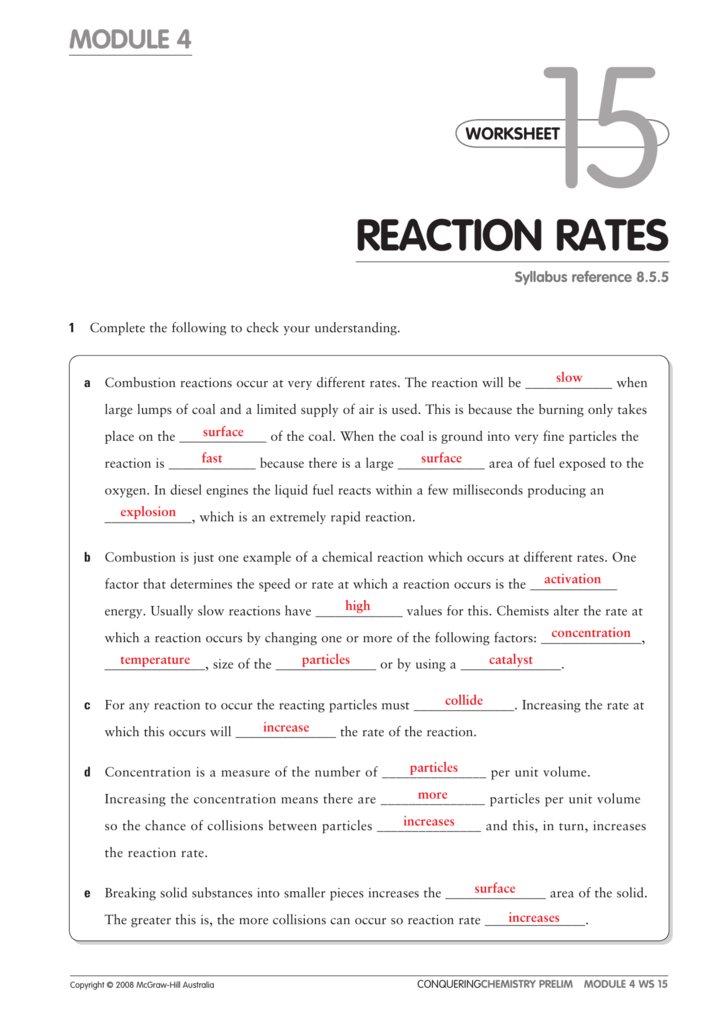
A Study Of Reaction _____ Is Called Chemical _____.
C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A student investigated the rate of reaction between calcium carbonate (marble chips) and. Give some examples of situations where we might want to decrease the rate of a particular reaction. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid.
Rates Of Reaction Some Reactions Occur Very Quickly, Like The Burning Of Petrol In Air In An Engine.
Other reactions can take a very long time, like the. Calculate the mean rate of reaction from 0 to 15 seconds. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear.