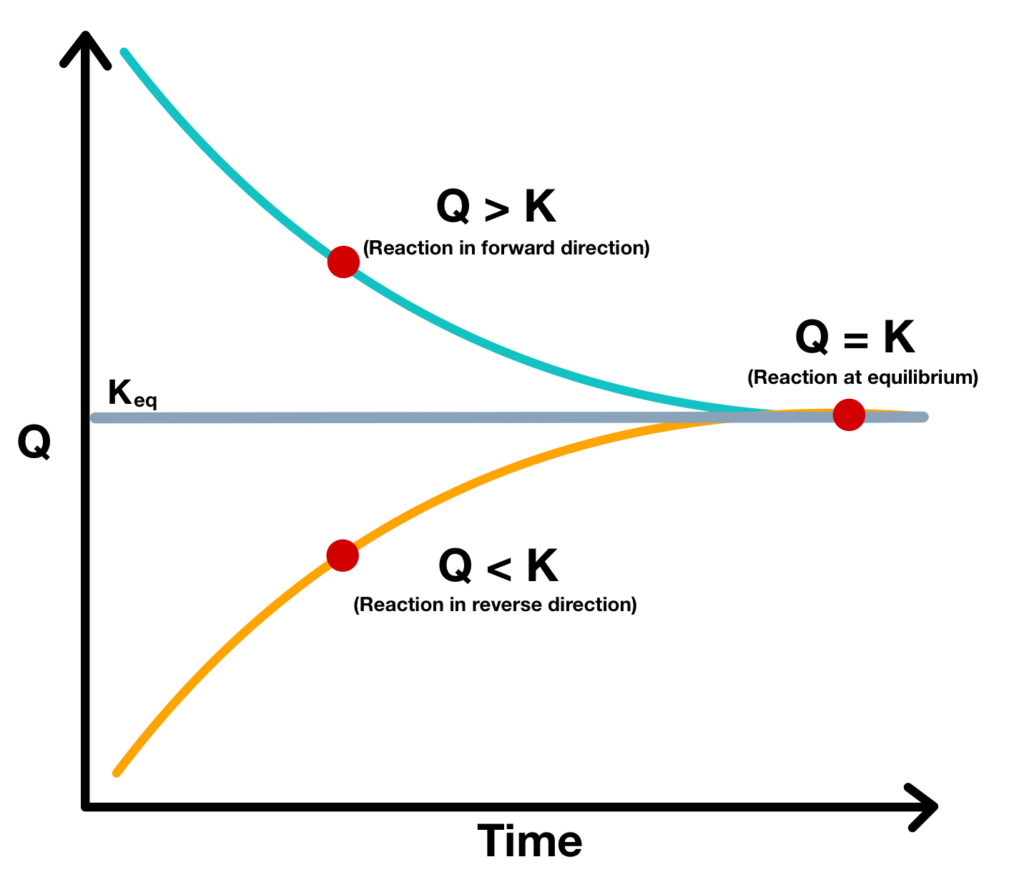
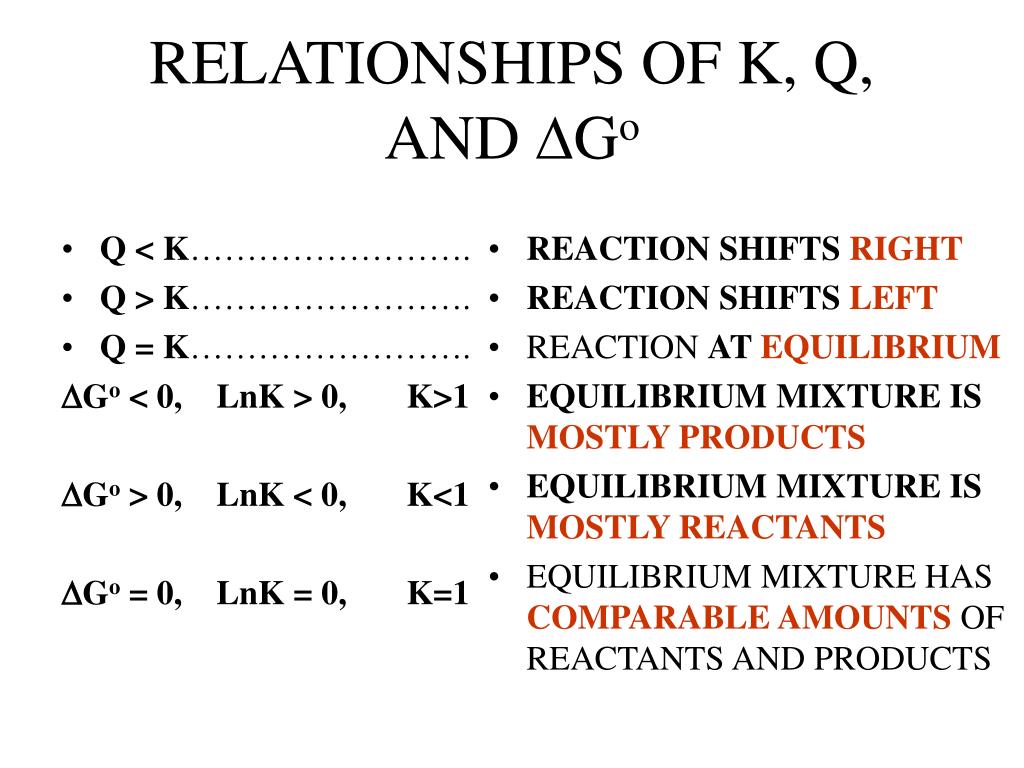
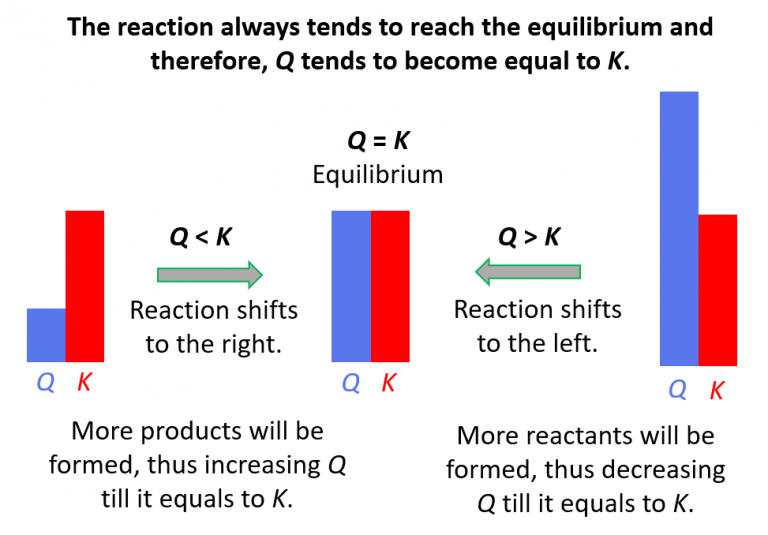
Relationship Between Q And K
Relationship Between Q And K - Q can be used to determine which direction a reaction will shift to reach equilibrium. K represents the equilibrium constant, which is. Q and k are used to describe the state of equilibrium in a chemical reaction. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. The equilibrium constant is denoted by the letter “k” in. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. If k > q, a reaction will proceed forward, converting.
The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. If k > q, a reaction will proceed forward, converting. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. Q and k are used to describe the state of equilibrium in a chemical reaction. K represents the equilibrium constant, which is. The equilibrium constant is denoted by the letter “k” in. Q can be used to determine which direction a reaction will shift to reach equilibrium.
Q and k are used to describe the state of equilibrium in a chemical reaction. K represents the equilibrium constant, which is. The equilibrium constant is denoted by the letter “k” in. Q can be used to determine which direction a reaction will shift to reach equilibrium. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. If k > q, a reaction will proceed forward, converting. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction.
The Difference between Q and Keq (Equilibrium) YouTube
Q can be used to determine which direction a reaction will shift to reach equilibrium. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. K represents the equilibrium constant, which is. Q and k are used to describe the state of equilibrium in a chemical reaction. The.
Establish relationship between Kp Kc?
The equilibrium constant is denoted by the letter “k” in. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. Q and k are used to describe the state of equilibrium in.
Reaction Quotient (Q) Equation, Calculation, Types, Units
The equilibrium constant is denoted by the letter “k” in. K represents the equilibrium constant, which is. Q can be used to determine which direction a reaction will shift to reach equilibrium. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. If k > q, a reaction.
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID
The equilibrium constant is denoted by the letter “k” in. Q can be used to determine which direction a reaction will shift to reach equilibrium. Q and k are used to describe the state of equilibrium in a chemical reaction. K represents the equilibrium constant, which is. If k > q, a reaction will proceed forward, converting.
What Is Kc In Chemistry slideshare
The equilibrium constant is denoted by the letter “k” in. Q can be used to determine which direction a reaction will shift to reach equilibrium. Q and k are used to describe the state of equilibrium in a chemical reaction. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. The main difference between.
Explain the Difference Between K Kp and Q LarryhasPark
If k > q, a reaction will proceed forward, converting. K represents the equilibrium constant, which is. Q and k are used to describe the state of equilibrium in a chemical reaction. Q can be used to determine which direction a reaction will shift to reach equilibrium. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction.
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
Q can be used to determine which direction a reaction will shift to reach equilibrium. The equilibrium constant is denoted by the letter “k” in. If k > q, a reaction will proceed forward, converting. Q and k are used to describe the state of equilibrium in a chemical reaction. The main difference between \(k\) and \(q\) is that \(k\).
Relationship between q k and q for different values of Δ (m = 4
The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. Q and k are used to describe the state of equilibrium in a chemical reaction. If k > q, a reaction will proceed forward, converting. Q can be used to determine which direction a reaction will shift to.
Reaction Quotient Q Chemistry Steps
Q can be used to determine which direction a reaction will shift to reach equilibrium. If k > q, a reaction will proceed forward, converting. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. K represents the equilibrium constant, which is. The main difference between \(k\) and \(q\) is that \(k\) describes a.
Reaction Quotient (K) and Equilibrium Constant (K) Problems & Examples
Q and k are used to describe the state of equilibrium in a chemical reaction. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. K represents the equilibrium constant, which is. The equilibrium constant is denoted by the letter “k” in. If k > q, a reaction will proceed forward, converting.
Q And K Are Used To Describe The State Of Equilibrium In A Chemical Reaction.
K represents the equilibrium constant, which is. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. If k > q, a reaction will proceed forward, converting. The equilibrium constant is denoted by the letter “k” in.
Q Can Be Used To Determine Which Direction A Reaction Will Shift To Reach Equilibrium.
The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction.