What Does Each Box In An Orbital Diagram Represent
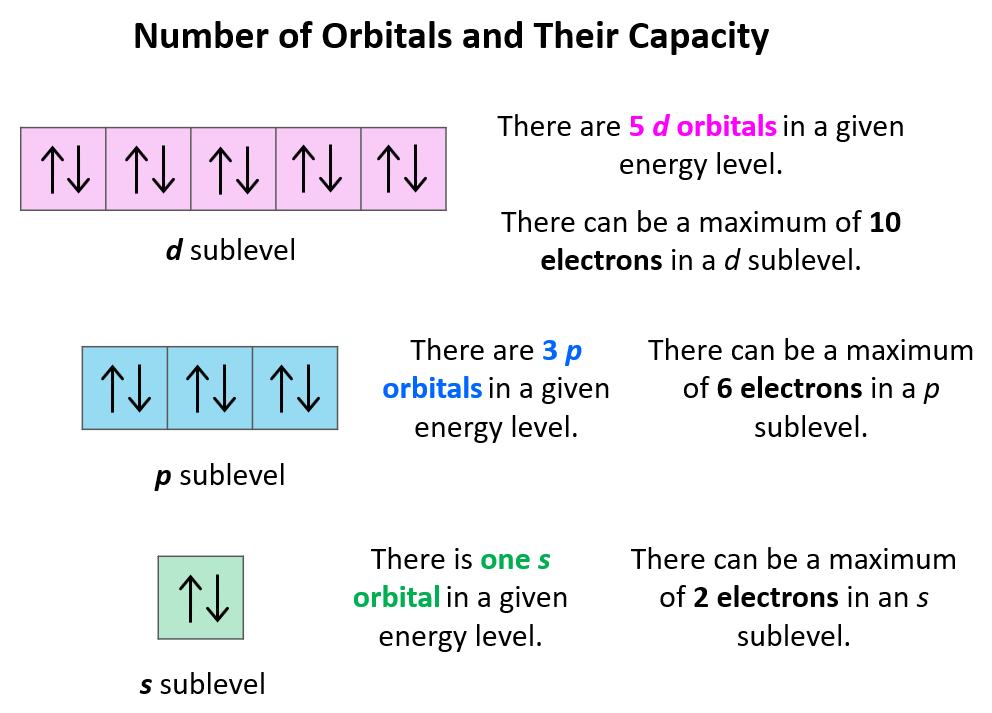
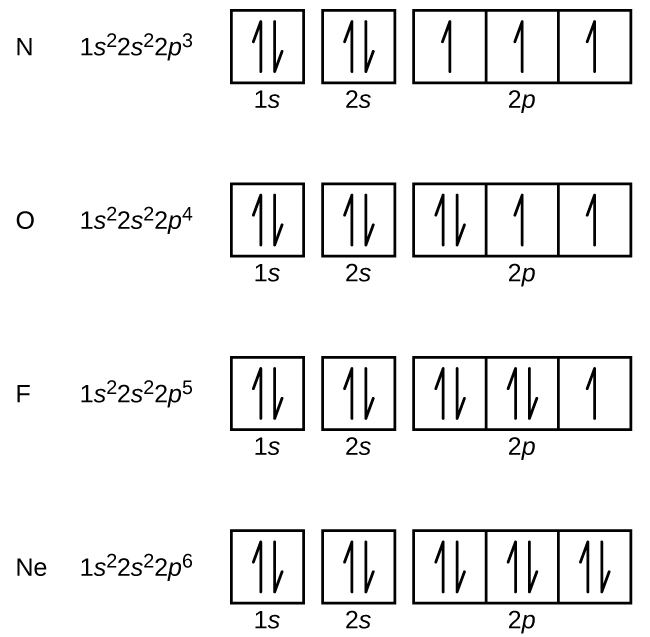
What Does Each Box In An Orbital Diagram Represent - Orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. The boxes are organized by principal quantum. In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital.
The boxes are organized by principal quantum. Orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons.
In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. Orbital diagram (orbital box diagram) : Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. The boxes are organized by principal quantum.
Chemistry Dec 3, 2018 P3 Challenge Today’s Objective ppt download
Orbital diagram (orbital box diagram) : In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each.
Orbital Diagram Worksheet Printable Word Searches
Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Orbital diagram (orbital box diagram) : In an orbital diagram, each orbital is represented by a box, with.
Orbital Diagram Worksheet Printable Word Searches
Orbital diagram (orbital box diagram) : Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. The boxes are organized by principal quantum. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. In an orbital diagram, each.
Orbital Box Diagram For Iron
The boxes are organized by principal quantum. Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Orbital diagram (orbital box diagram) : In an orbital diagram, each.
Electron Configuration Diagram Orbitals
Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. The boxes are organized by principal quantum. Orbital diagram (orbital box diagram) : Study with quizlet and memorize flashcards containing terms like.
Orbital Diagrams Chemistry Steps
Orbital diagram (orbital box diagram) : Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with.
SOLVED(a) What are "valence electrons"? (b) What are "core electrons
Orbital diagram (orbital box diagram) : The boxes are organized by principal quantum. Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. In an orbital diagram, each.
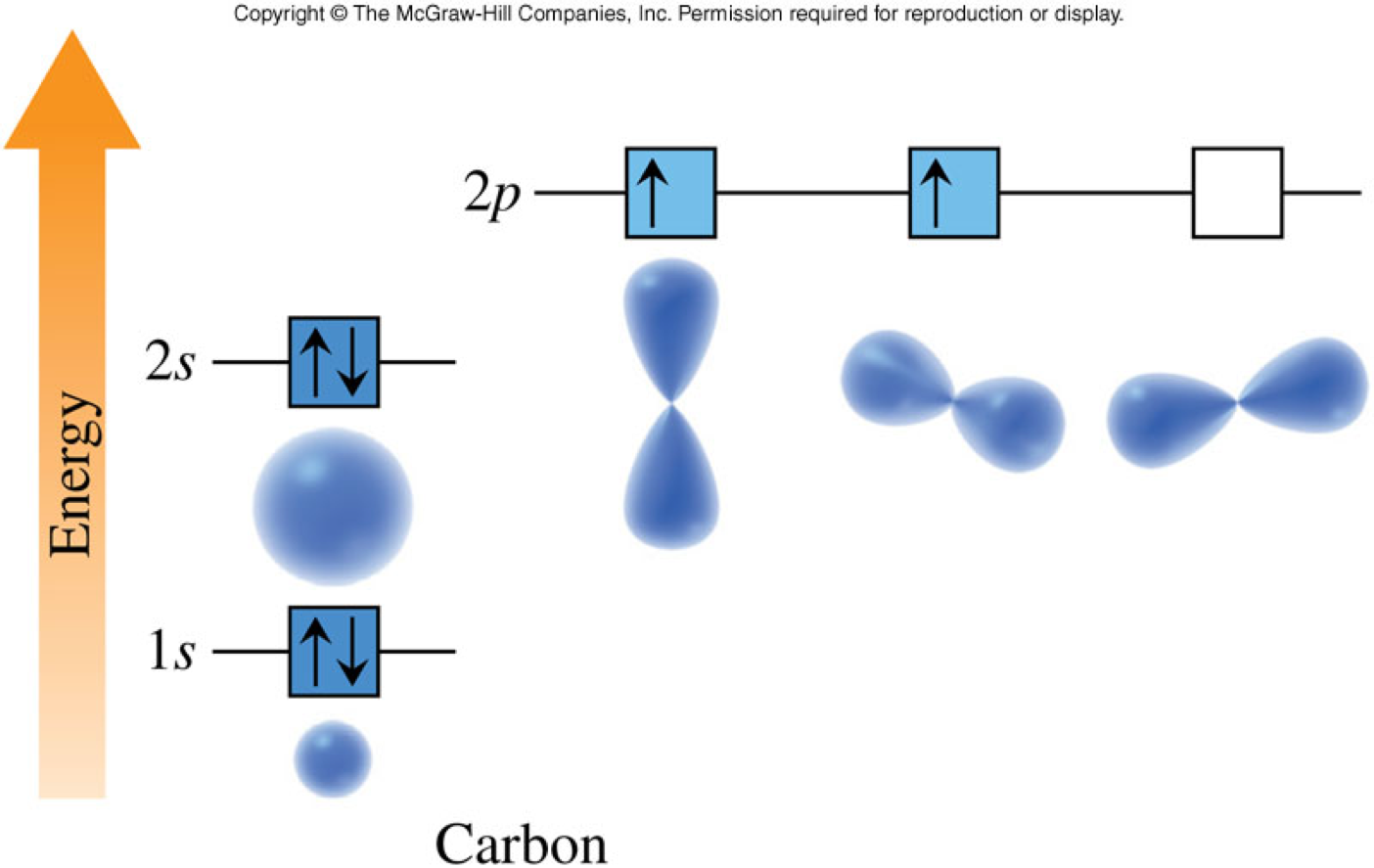
Which is the correct orbital diagram for carbon? ОА. 1s 2s 2p OB. 1s 2s
Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. Orbital diagram (orbital box diagram) : In an orbital diagram, each orbital is represented by a box, with.
Which neutral element does the orbital diagram represent?
Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital. In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Orbital diagram.
36 what does each box in an orbital diagram represent Diagram Online
The boxes are organized by principal quantum. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Orbital diagram (orbital box diagram) : In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. Study with quizlet and memorize flashcards containing terms like.
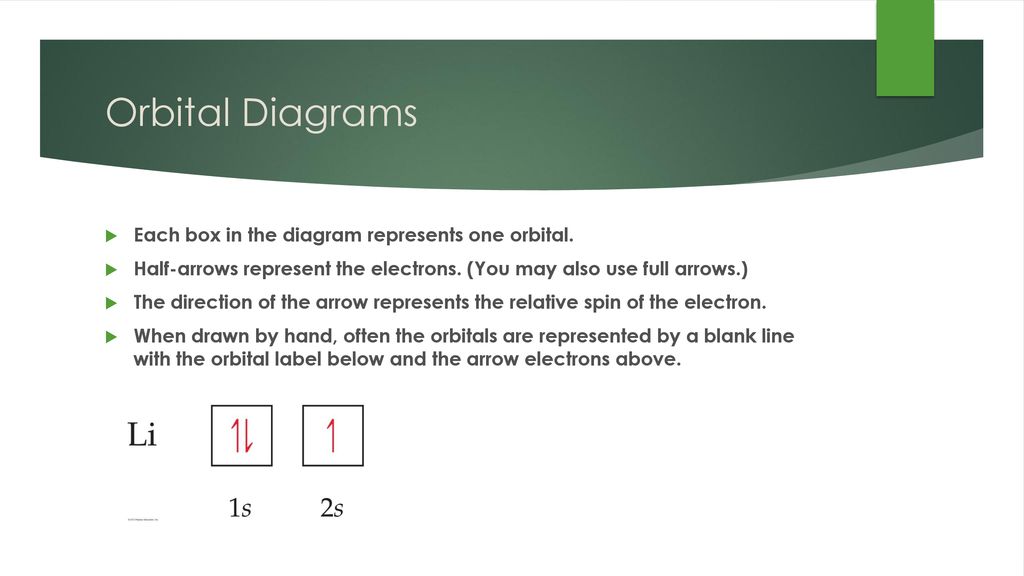
In An Orbital Diagram, Each Orbital Is Represented By A Box, With Arrows Denoting The Electrons.
Orbital diagram (orbital box diagram) : The boxes are organized by principal quantum. Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p. Study with quizlet and memorize flashcards containing terms like valence electrons, core electrons, what does each box in an orbital.


:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)