What Intermolecular Forces Are Present In Nh3
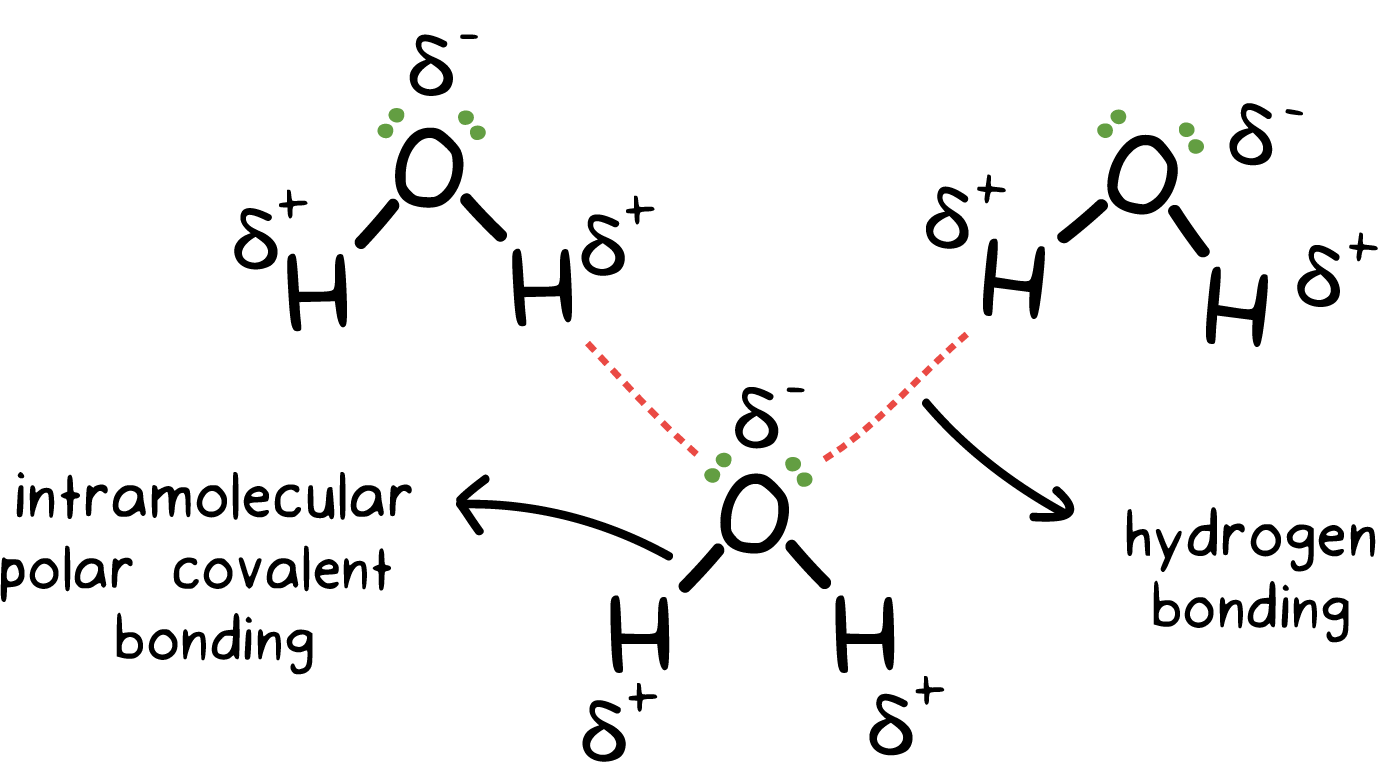
What Intermolecular Forces Are Present In Nh3 - The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. The hydrogen bonds are many magnitudes. London dispersion and hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. Every molecule experiences london dispersion as an intermolecular force. In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces.
In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. The hydrogen bonds are many magnitudes. Every molecule experiences london dispersion as an intermolecular force. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. London dispersion and hydrogen bonds.
In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. The hydrogen bonds are many magnitudes. London dispersion and hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force.
Intermolecular Forces in Chemistry
In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. London dispersion and hydrogen bonds. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. Every molecule experiences london dispersion as an intermolecular force.
Image result for intermolecular forces Chemie
The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. London dispersion and hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. Every molecule experiences london dispersion as an intermolecular force.
Solved The intermolecular forces present in NH3 include
London dispersion and hydrogen bonds. The hydrogen bonds are many magnitudes. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen.
H2S Intermolecular Forces (Strong or Weak) Techiescientist
The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. The hydrogen bonds are many magnitudes. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. London dispersion and hydrogen bonds.
SOLVED NH3, has the same molecular shape as PCl3. Which intermolecular
The hydrogen bonds are many magnitudes. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces.
Intermolecular Forces for NH3 (Ammonia) YouTube
In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. London dispersion and hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen.
intermolecular force of nh3
The hydrogen bonds are many magnitudes. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. Every molecule experiences london dispersion as an intermolecular force. London dispersion and hydrogen bonds. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds.
vapor pressure intermolecular forces
In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. The hydrogen bonds are many magnitudes. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. London dispersion and hydrogen bonds.
intermolecular vs. intramolecular forces Diagram Quizlet
In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. The hydrogen bonds are many magnitudes. London dispersion and hydrogen bonds.
SOLVED Identify the intermolecular forces present in each of these
Every molecule experiences london dispersion as an intermolecular force. In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. London dispersion and hydrogen bonds. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds.
The Types Of Intermolecular Forces Present In Ammonia, Or Nh3, Are Hydrogen Bonds.
In ammonia (nh3), the intermolecular forces present are hydrogen bonding and london dispersion forces. The hydrogen bonds are many magnitudes. Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen.