What Is The Charge On The Iron Ion In Fe2S3
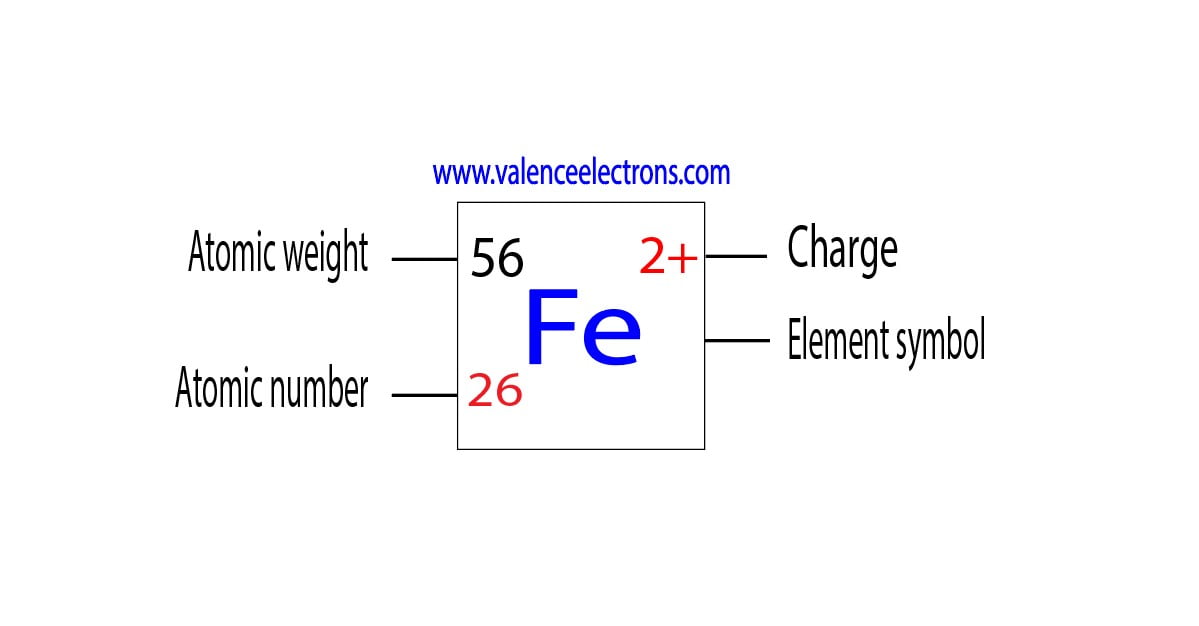
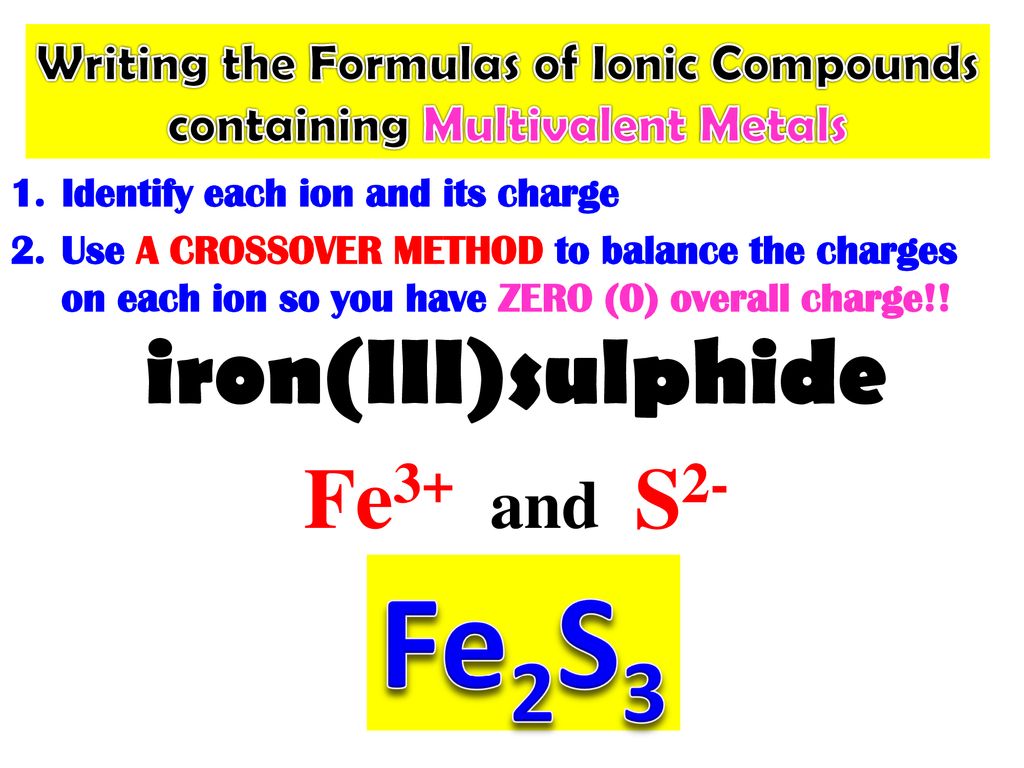
What Is The Charge On The Iron Ion In Fe2S3 - Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. It is a solid, black powder that. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. The correct formula for iron(iii) sulfide is fe2s3. In an ionic molecule the formula is. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. Your solution’s ready to go! Essentially, the formula fe2s3 indicates that the. What is the charge on the iron in fe2s3?
What is the charge on the iron in fe2s3? Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. The correct formula for iron(iii) sulfide is fe2s3. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. Your solution’s ready to go! In an ionic molecule the formula is. Essentially, the formula fe2s3 indicates that the. It is a solid, black powder that.
It is a solid, black powder that. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Essentially, the formula fe2s3 indicates that the. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. In an ionic molecule the formula is. What is the charge on the iron in fe2s3? Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. Your solution’s ready to go! The correct formula for iron(iii) sulfide is fe2s3.
Ionic Charge for Iron (Fe) YouTube
Essentially, the formula fe2s3 indicates that the. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. It is a solid, black powder that. What is the charge.
Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry
In an ionic molecule the formula is. Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. Essentially, the formula fe2s3 indicates that the. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. In the ionic compound fes (iron sulfide), the.
Question Video Deducing the Ionic Formula of an Ionic Compound Where
Your solution’s ready to go! Essentially, the formula fe2s3 indicates that the. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. In an ionic molecule the formula is. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two.
How Many Protons, Neutrons and Electrons Does Iron Have?
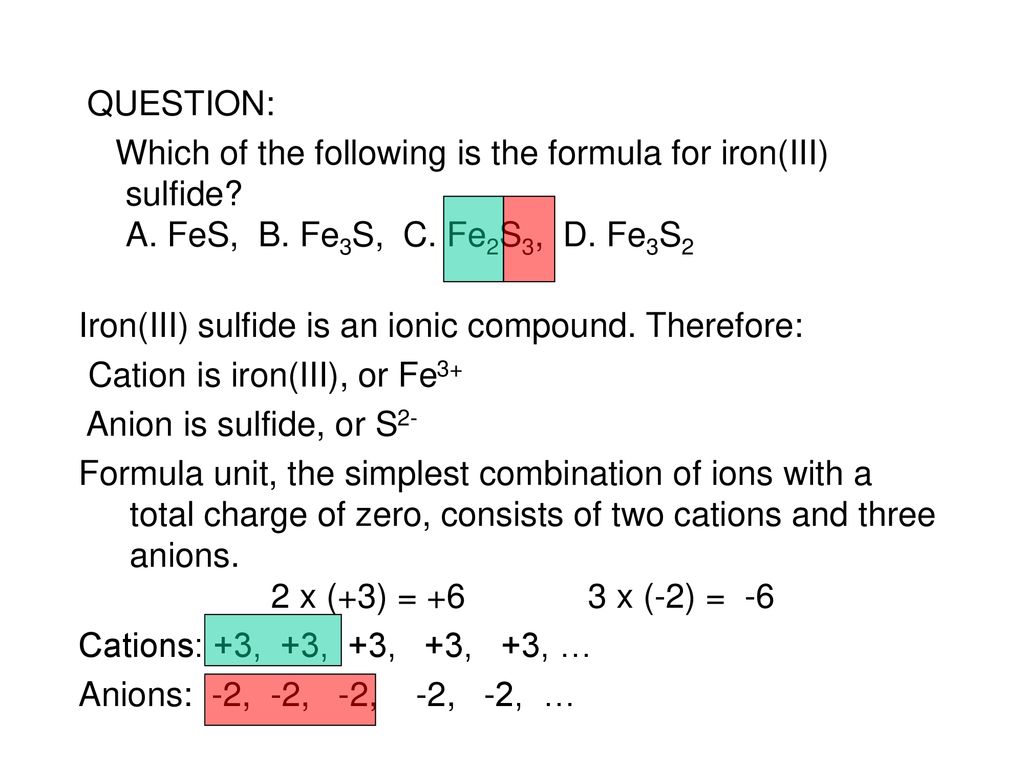
The correct formula for iron(iii) sulfide is fe2s3. What is the charge on the iron in fe2s3? It is a solid, black powder that. Your solution’s ready to go! Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides.
3.2 Names and Formulas of Ionic Compounds ppt download
Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Your solution’s ready to go! What is the charge on the iron in fe2s3? In the ionic compound.
What Is the Charge on the Iron Ion in Fe2s3
What is the charge on the iron in fe2s3? Your solution’s ready to go! Essentially, the formula fe2s3 indicates that the. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has.
How to Write the Net Ionic Equation for Fe2S3 + HBr = H2S + FeBr3 YouTube
In an ionic molecule the formula is. Essentially, the formula fe2s3 indicates that the. It is a solid, black powder that. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. The correct formula for iron(iii) sulfide is fe2s3.
What Is the Charge on the Iron Ion in Fe2s3
The correct formula for iron(iii) sulfide is fe2s3. It is a solid, black powder that. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. What is the charge on the iron in fe2s3? Your solution’s ready to go!
Nomenclature Part I PO43 phosphate ion HC2H3O2 Acetic acid C2H3O2
The correct formula for iron(iii) sulfide is fe2s3. What is the charge on the iron in fe2s3? Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. Your solution’s ready to go!
What Is Fe3 And Fe2? Quick Answer
Your solution’s ready to go! It is a solid, black powder that. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Essentially, the formula fe2s3 indicates that.
Explanation The Roman Numeral (Iii) In The Name Iron(Iii) Sulfide Indicates The Charge Of The Iron Ion.
In an ionic molecule the formula is. Your solution’s ready to go! It is a solid, black powder that. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons.
What Is The Charge On The Iron In Fe2S3?
Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Essentially, the formula fe2s3 indicates that the. The correct formula for iron(iii) sulfide is fe2s3.





+iron+(III)+chloride.jpg)
