What Is The Correct Lewis Structure For N2
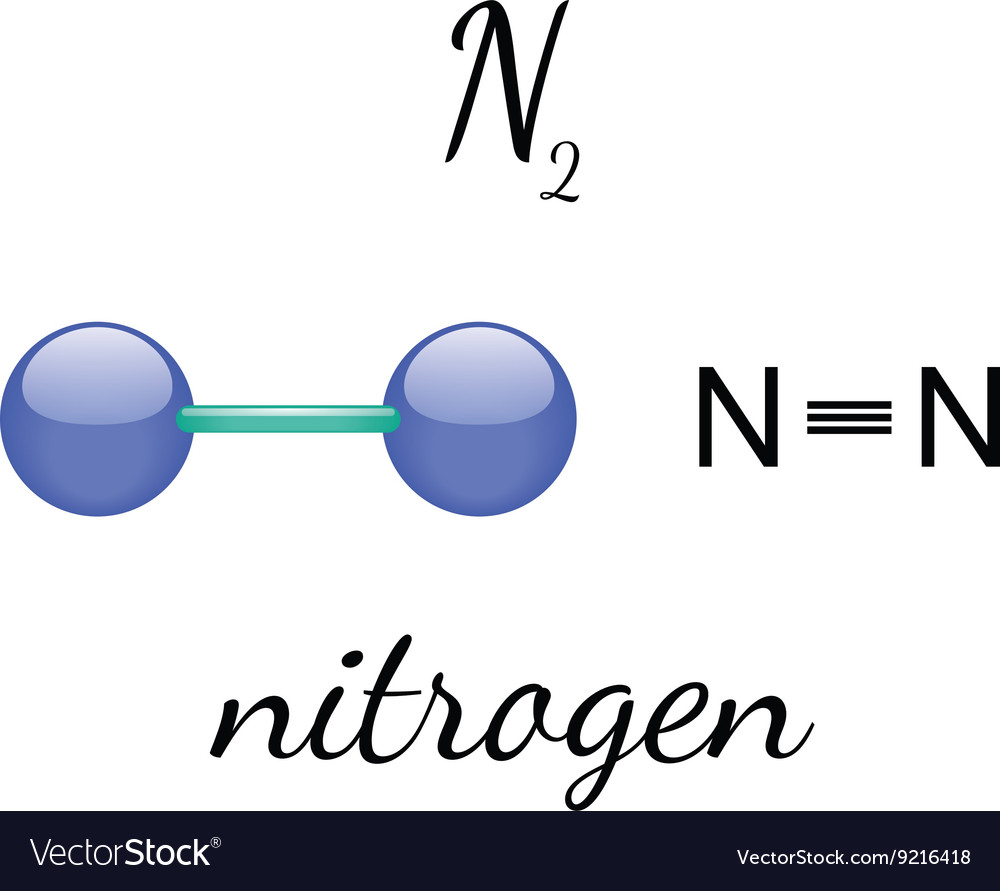
What Is The Correct Lewis Structure For N2 - Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. The correct lewis structure for n2 is :n = n:. The correct lewis structure for n2 is :
The correct lewis structure for n2 is : Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. The correct lewis structure for n2 is :n = n:.
The correct lewis structure for n2 is :n = n:. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. The correct lewis structure for n2 is : D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen.
Nitrogen And Hydrogen Electron Dot Diagram
The correct lewis structure for n2 is : D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. The correct lewis structure for n2 is :n = n:.
N2 Lewis Structure N2 Lewis Dot Structure Nitrogen Gas Lewis
D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. The correct lewis structure for n2 is :n = n:. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. The correct lewis structure for n2 is :
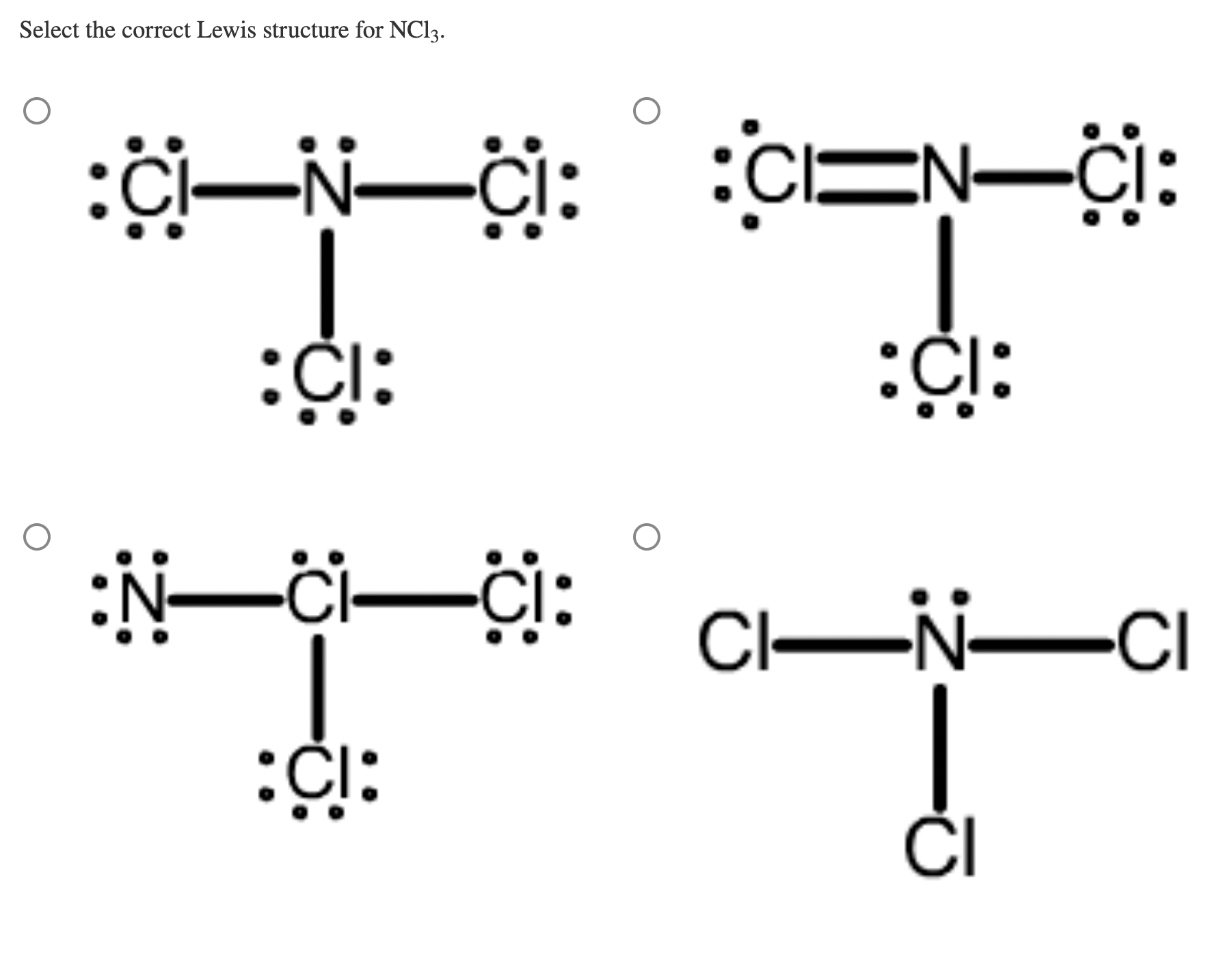
Solved Select the correct Lewis structure for NCl3
The correct lewis structure for n2 is : The correct lewis structure for n2 is :n = n:. D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost.
Which of the following is the correct Lewis structure for nitrogen gas
Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. The correct lewis structure for n2 is : The correct lewis structure for n2 is :n = n:.
What Is The Electron Dot Diagram For Nitrogen
The correct lewis structure for n2 is :n = n:. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. The correct lewis structure for n2 is :
(Solved) 9 What is the correct Lewis structure for N2? A) NN B) D)N
The correct lewis structure for n2 is : D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. The correct lewis structure for n2 is :n = n:. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost.
Lewis Dot Structure For N2 Draw Easy
The correct lewis structure for n2 is : The correct lewis structure for n2 is :n = n:. D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost.
N2 Lewis Dot Structure
The correct lewis structure for n2 is : The correct lewis structure for n2 is :n = n:. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen.
Electron Dot Diagram For N2
D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. The correct lewis structure for n2 is :n = n:. The correct lewis structure for n2 is :
[Solved] Please answer and draw the correct Lewis structure. Determine
D) n ≡ n :, which shows a triple bond between the two nitrogen atoms, ensuring that each nitrogen. The correct lewis structure for n2 is :n = n:. Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost. The correct lewis structure for n2 is :
D) N ≡ N :, Which Shows A Triple Bond Between The Two Nitrogen Atoms, Ensuring That Each Nitrogen.
The correct lewis structure for n2 is :n = n:. The correct lewis structure for n2 is : Both nitrogen atoms form a triple bond, sharing three electrons each to fill their outermost.