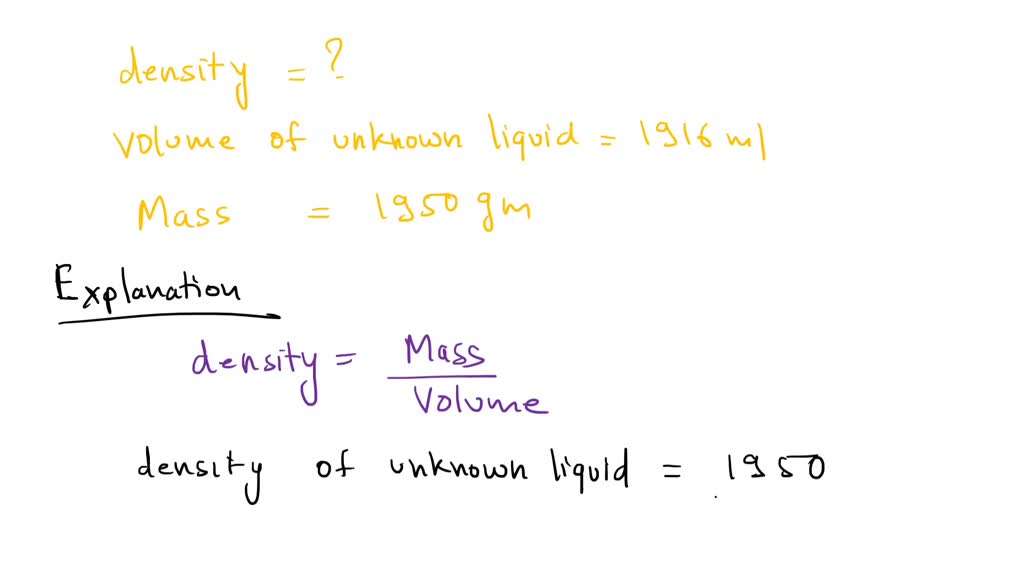What Is The Density Of Glycerol In G Cm3
What Is The Density Of Glycerol In G Cm3 - Density of aqueous solutions at 20°c, given as g/cm 3: Density of glycerol is equal to 1 261.3 kg/m³; Glycerol is a syrupy liquid often used in cosmetics and soaps. The density of glycerin is 1.26 g/cm3. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. What is the mass in g of 417 ml of ethylene glycol? Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³). Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with.
Glycerol is a syrupy liquid often used in cosmetics and soaps. Density of aqueous solutions at 20°c, given as g/cm 3: 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. The density of glycerin is 1.26 g/cm3. Density of glycerol is equal to 1 261.3 kg/m³; If not provided, you may need to look it up in a reliable. What is the volume in l of 4.1. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with.
What is the volume in l of 4.1. 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Density of aqueous solutions at 20°c, given as g/cm 3: What is the mass in g of 417 ml of ethylene glycol? Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: If not provided, you may need to look it up in a reliable. Density of glycerol is equal to 1 261.3 kg/m³; Glycerol is a syrupy liquid often used in cosmetics and soaps. Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³). Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with.
[Solved] A chemistry student needs 30.0 g of glycerol for an experiment
Density of glycerol is equal to 1 261.3 kg/m³; The density of glycerin is 1.26 g/cm3. What is the volume in l of 4.1. What is the mass in g of 417 ml of ethylene glycol? Glycerol is a syrupy liquid often used in cosmetics and soaps.
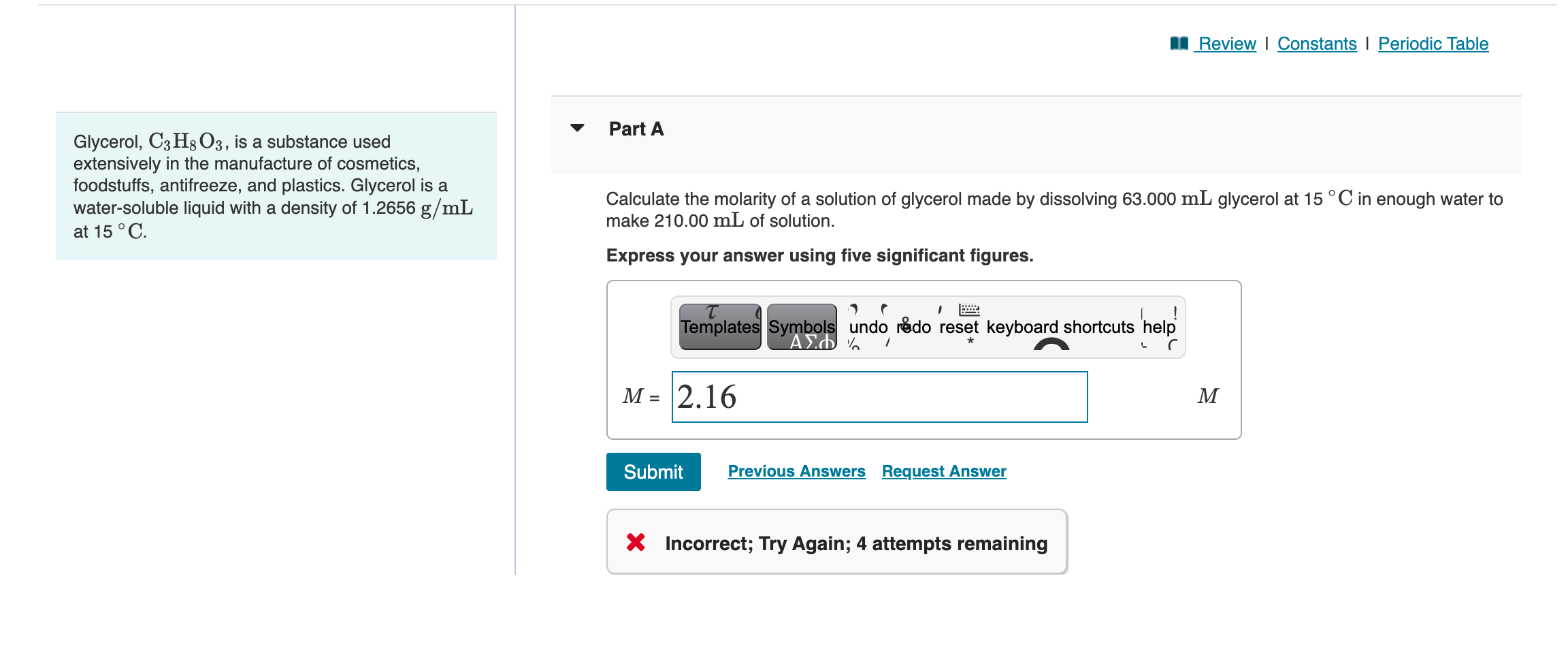
Solved Glycerol, C3H8O3, is a substance used Part A
Density of glycerol is equal to 1 261.3 kg/m³; What is the volume in l of 4.1. 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. What is the mass in g of 417 ml of ethylene glycol? If not provided, you may need to look it up in a reliable.
Glycerol monostearate Henan Eshine Chemicals Co.,Ltd
The density of glycerin is 1.26 g/cm3. Density of aqueous solutions at 20°c, given as g/cm 3: If not provided, you may need to look it up in a reliable. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution.
SOLVED What is the density of 2 glycerol, given that the density of
20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. What is the mass in g of 417 ml of ethylene glycol? What is the volume in l of 4.1. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density.
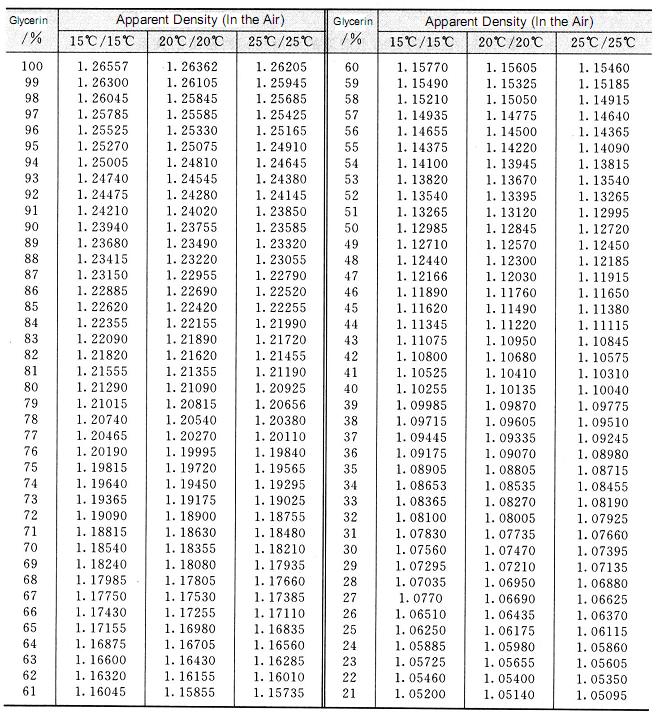
Densities (g cm 3 ) for glycerol + water mixtures at various
Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: What is the mass in g of 417 ml of ethylene glycol? Density of aqueous solutions at 20°c, given as g/cm 3: 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. The density of glycerin is.
SOLVED Next the chemist measures the volume of the unknown liquid as 0
Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: Density of glycerol is equal to 1 261.3 kg/m³; What is the mass in g of 417 ml of ethylene glycol? Glycerol is a syrupy liquid often used in cosmetics and soaps.
Solved A 20.0 mL sample of glycerol has a mass of 25.2
Density of glycerol is equal to 1 261.3 kg/m³; Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. What is the mass in g of 417 ml of ethylene glycol? The density of glycerin is 1.26 g/cm3.
Glycerol density gradient centrifugation to determine PKM2 oligomers
20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Density of glycerol is equal to 1 261.3 kg/m³; Glycerol is a syrupy liquid often used in cosmetics and soaps. What is the volume in l of 4.1.
Density of Glycerol {🪨 2022 update}
Density of glycerol is equal to 1 261.3 kg/m³; Density of aqueous solutions at 20°c, given as g/cm 3: The density of glycerin is 1.26 g/cm3. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. If not provided, you may need to look it up in a reliable.
Glycerin’s relative density Glycerin Refinery Equipment
20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. The density of glycerin is 1.26 g/cm3. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. What is the volume in l of 4.1. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3.
Typically, The Density Of Glycerol Is Provided In Units Of Grams Per Cubic Centimeter (G/Cm³).
Glycerol is a syrupy liquid often used in cosmetics and soaps. 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. What is the mass in g of 417 ml of ethylene glycol?
Density Of Aqueous Solutions At 20°C, Given As G/Cm 3:
What is the volume in l of 4.1. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: If not provided, you may need to look it up in a reliable. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3.
Density Of Glycerol Is Equal To 1 261.3 Kg/M³;
The density of glycerin is 1.26 g/cm3.