What Is The Electron Configuration For V2
What Is The Electron Configuration For V2 - The atomic number of vanadium (v) is 23. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The electronic configuration of vanadium can be.
The electronic configuration of vanadium can be. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The atomic number of vanadium (v) is 23.
The atomic number of vanadium (v) is 23. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The electronic configuration of vanadium can be.
Electron Configuration Definition, Orbits and Orbitals
1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The electronic configuration of vanadium can be. The atomic number of vanadium (v) is 23.
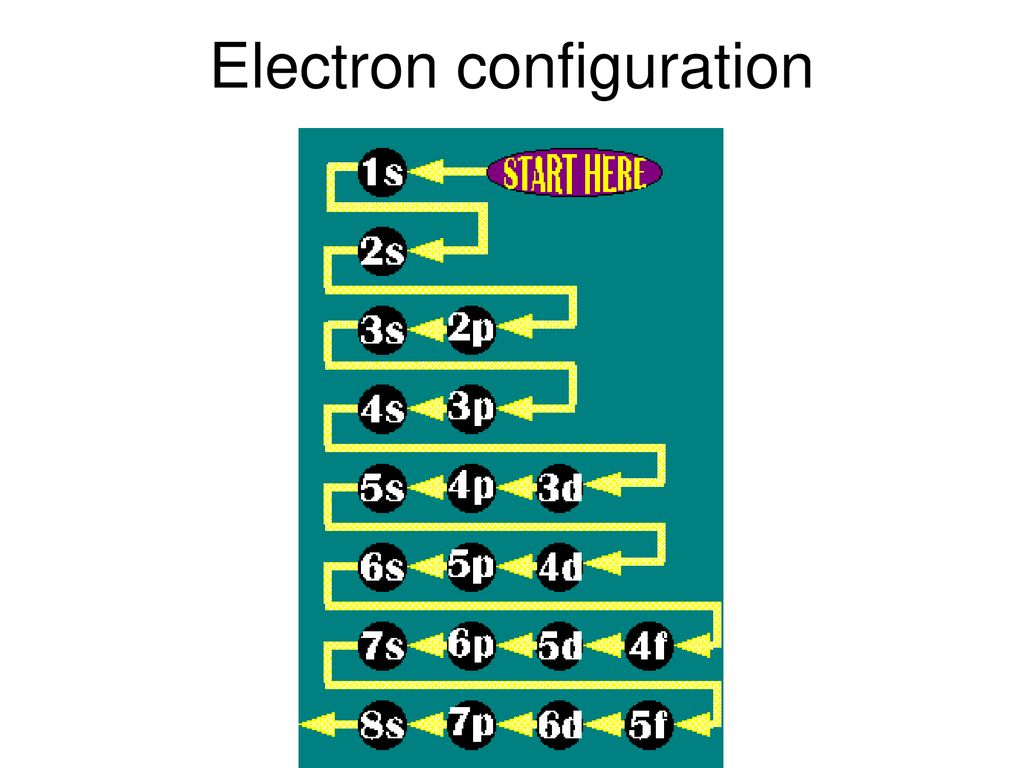
Electron configuration ppt download
1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The atomic number of vanadium (v) is 23. The electronic configuration of vanadium can be.
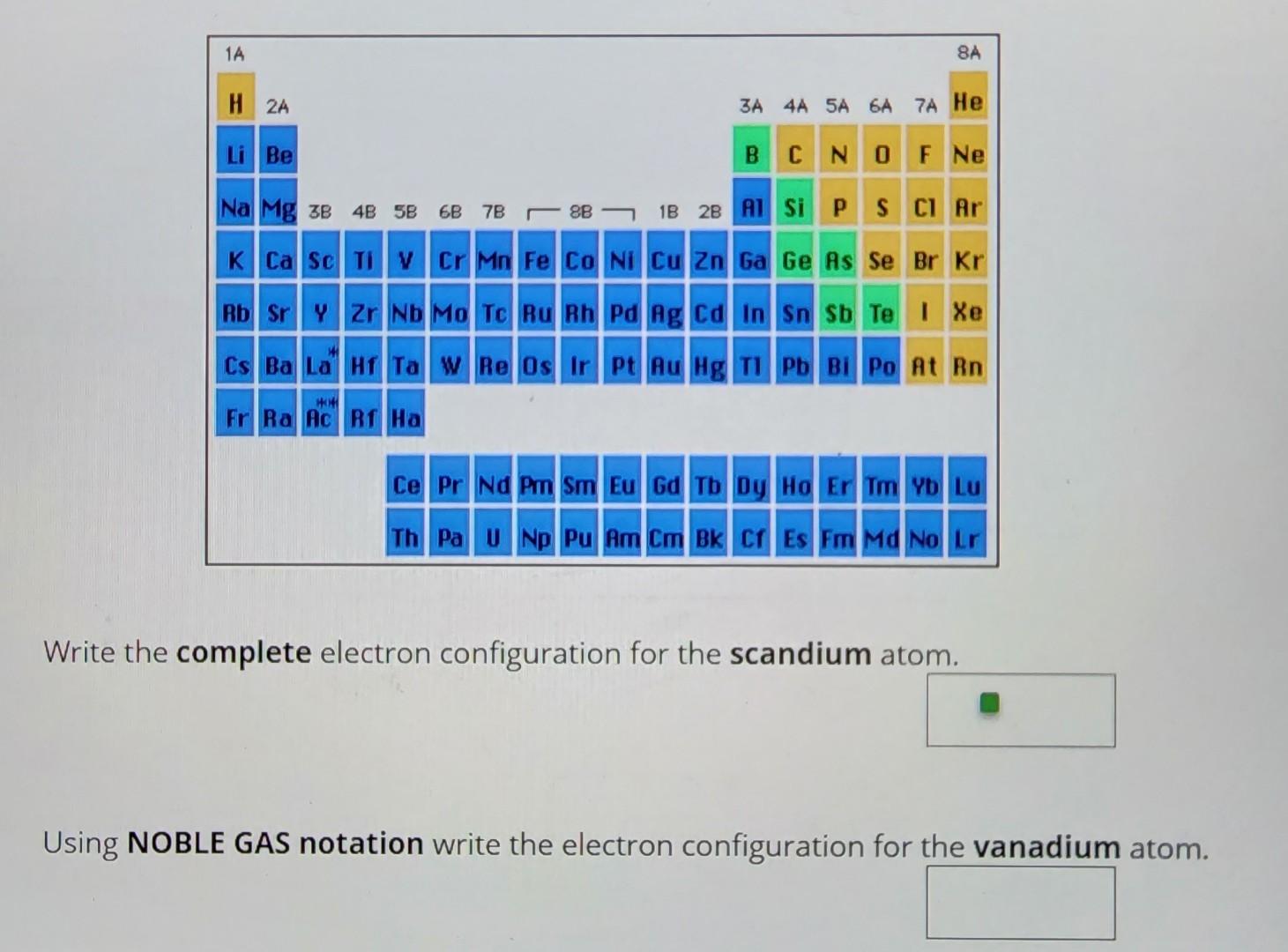
Solved Write the complete electron configuration for the
The electronic configuration of vanadium can be. The atomic number of vanadium (v) is 23. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons.
Question Video Identifying the Electronic Configuration of the
The atomic number of vanadium (v) is 23. The electronic configuration of vanadium can be. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons.
Electron Confiuration Practice WS KEY Electron Configuration Practice
1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The electronic configuration of vanadium can be. The atomic number of vanadium (v) is 23.
Electron Configuration 1939799 G9ISRScience
The electronic configuration of vanadium can be. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The atomic number of vanadium (v) is 23.
Free Printable Electron Configuration Worksheets
The electronic configuration of vanadium can be. The atomic number of vanadium (v) is 23. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons.
10 Surprising Facts About Electron Configuration Notation
The atomic number of vanadium (v) is 23. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The electronic configuration of vanadium can be.
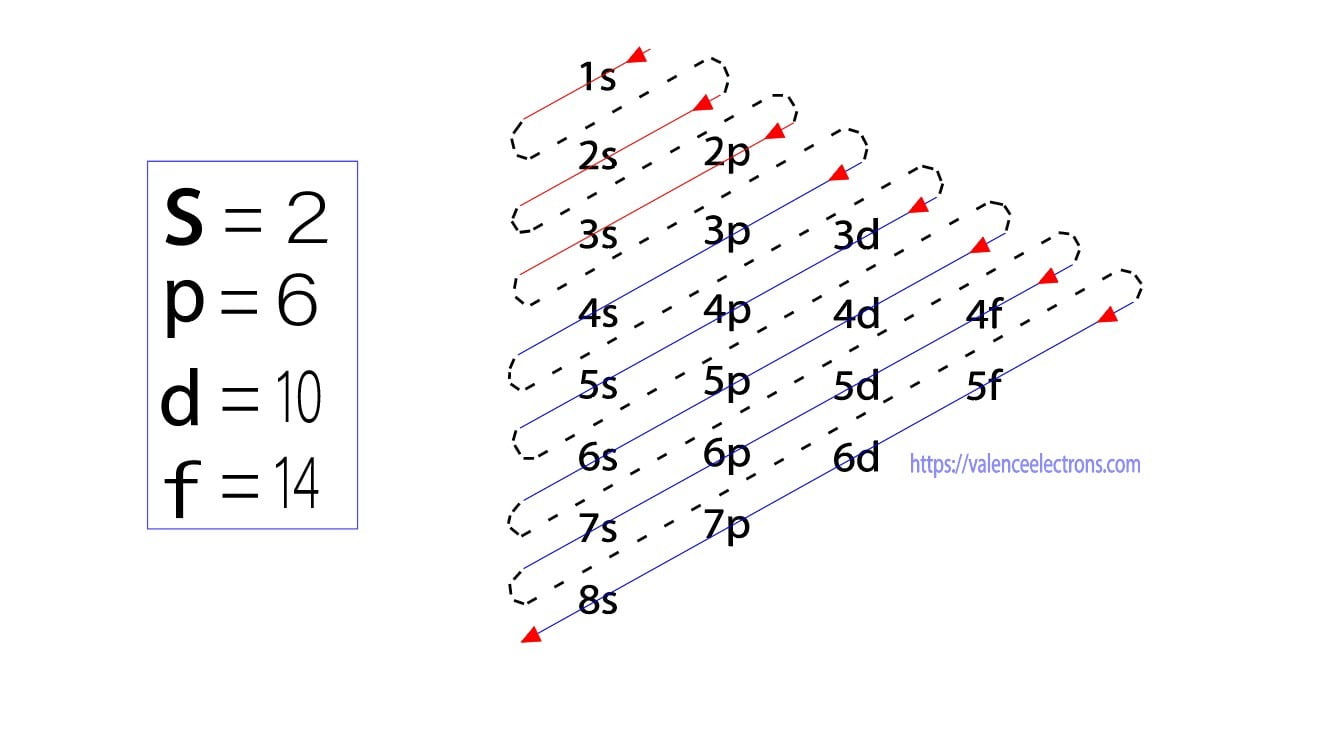
List of Electron Configuration Chart of All Elements [PDF]
The electronic configuration of vanadium can be. The atomic number of vanadium (v) is 23. 1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons.
The Electronic Configuration Of Vanadium Can Be.
1s^ {2} 2s^ {2} 2p^ {6} 3s^ {2} 3p^ {6} 3d^ {3} 1s22s22p63s23p63d3 loses 2 electrons. The atomic number of vanadium (v) is 23.



![List of Electron Configuration Chart of All Elements [PDF]](https://iperiodictable.com/wp-content/uploads/2021/02/Electron-Configuration-Chart-2-791x1024.jpg)
