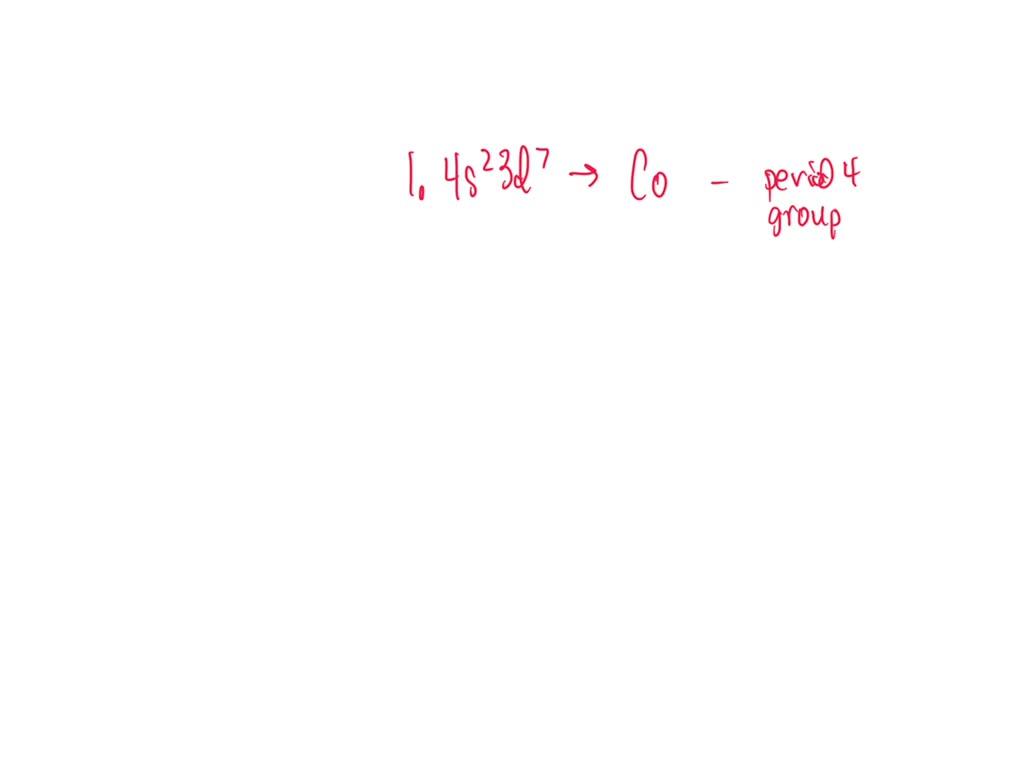What Is The Element With An Electron Configuration Of 1S22S22P63S23P64S23D7
What Is The Element With An Electron Configuration Of 1S22S22P63S23P64S23D7 - The number of protons must be the same as the element is electrically neutral. What element has the electron configuration 1s22s22p23s23p2? Just add up the electrons. There are 2 steps to solve this one. Remember that for neutral atoms,. The element with this electron configuration is carbon (c). Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. Then number of protons =. If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration.
Remember that for neutral atoms,. There are 2 steps to solve this one. What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. Then number of protons =. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. Just add up the electrons. The element with this electron configuration is carbon (c). What element has the electron configuration 1s22s22p23s23p2?
Remember that for neutral atoms,. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? Just add up the electrons. The number of protons must be the same as the element is electrically neutral. The element with this electron configuration is carbon (c). Then number of protons =. What element has the electron configuration 1s22s22p23s23p2? The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7.
Electron Configuration of Elements Chemistry Periodic Table
Remember that for neutral atoms,. There are 2 steps to solve this one. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. The element with this electron configuration is carbon (c). Then number of protons =.
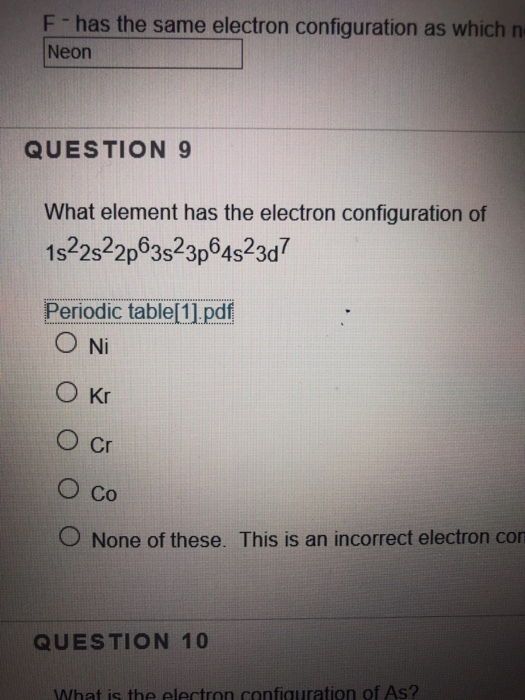
Solved F has the same electron configuration as which n
What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? The element with this electron configuration is carbon (c). Remember that for neutral atoms,. There are 2 steps to solve this one. Then number of protons =.
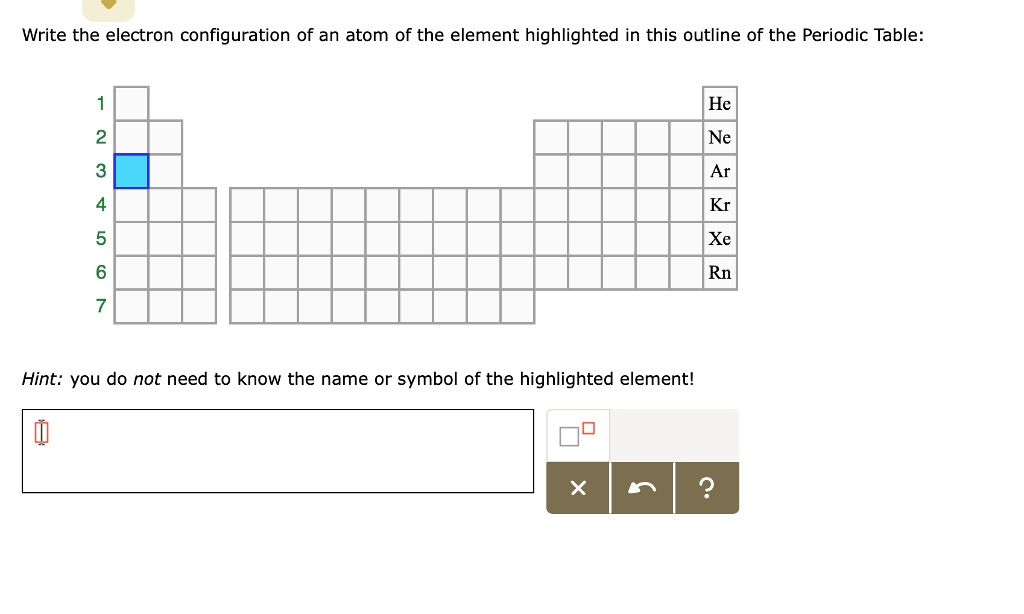
use the periodic table to identify the element indicated by each
Just add up the electrons. What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? The element with this electron configuration is carbon (c). The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. Then number of protons =.
Electronic Configuration Antimony Learn Important Terms and Concepts
If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. There are 2 steps to solve this one. The element with this electron configuration is carbon (c). The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ?
[Solved] Which element has the following electron configuration
The number of protons must be the same as the element is electrically neutral. The element with this electron configuration is carbon (c). Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. There are 2 steps to solve.
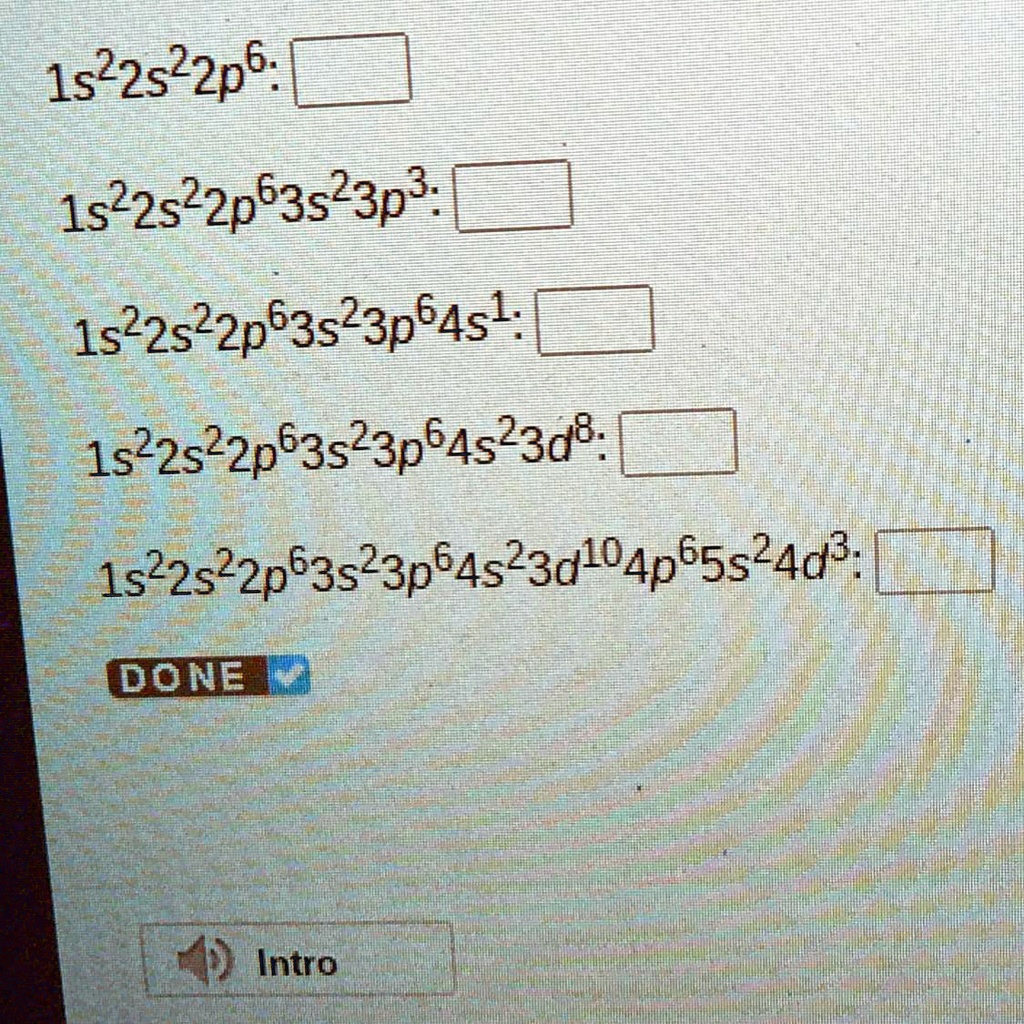
5.2 Electronic Structure of Atoms (Electron Configurations
Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. Remember that for neutral atoms,. Then number of protons =. What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? There are 2 steps to solve this one.
1.5 Electronic Structure of Atoms (Electron Configurations)
The number of protons must be the same as the element is electrically neutral. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. Then number of protons =. Just add up the electrons.
Electron Configuration Of Arsenic
The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. Remember that for neutral atoms,. There are 2 steps to solve this one. The element with this electron configuration is carbon (c). What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ?
write the electron configuration of an atom of the element highlighted
There are 2 steps to solve this one. The number of protons must be the same as the element is electrically neutral. Just add up the electrons. What element has the electron configuration 1s22s22p23s23p2? Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.
(1) The element with an electron configuration of 1s22s22p63s23p64s23d7
The number of protons must be the same as the element is electrically neutral. The element with this electron configuration is carbon (c). Just add up the electrons. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. What.
What Element Has The Ground State Electron Configuration 1S22S22P63S23P64S23D7 ?
Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. There are 2 steps to solve this one. Just add up the electrons. The element with this electron configuration is carbon (c).
The Number Of Protons Must Be The Same As The Element Is Electrically Neutral.
Remember that for neutral atoms,. What element has the electron configuration 1s22s22p23s23p2? The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration.