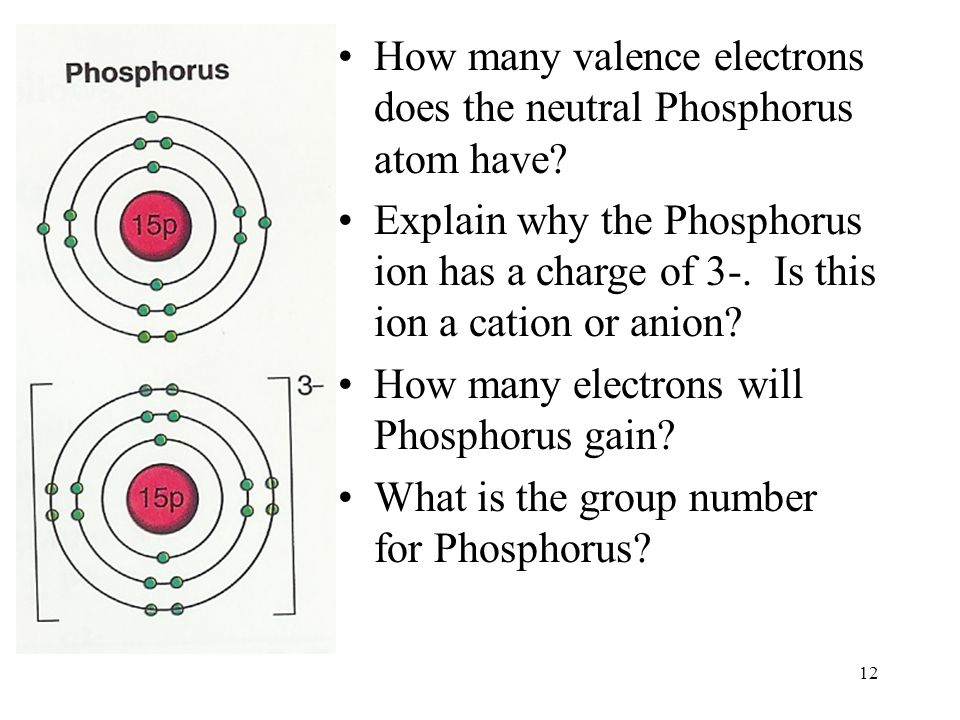
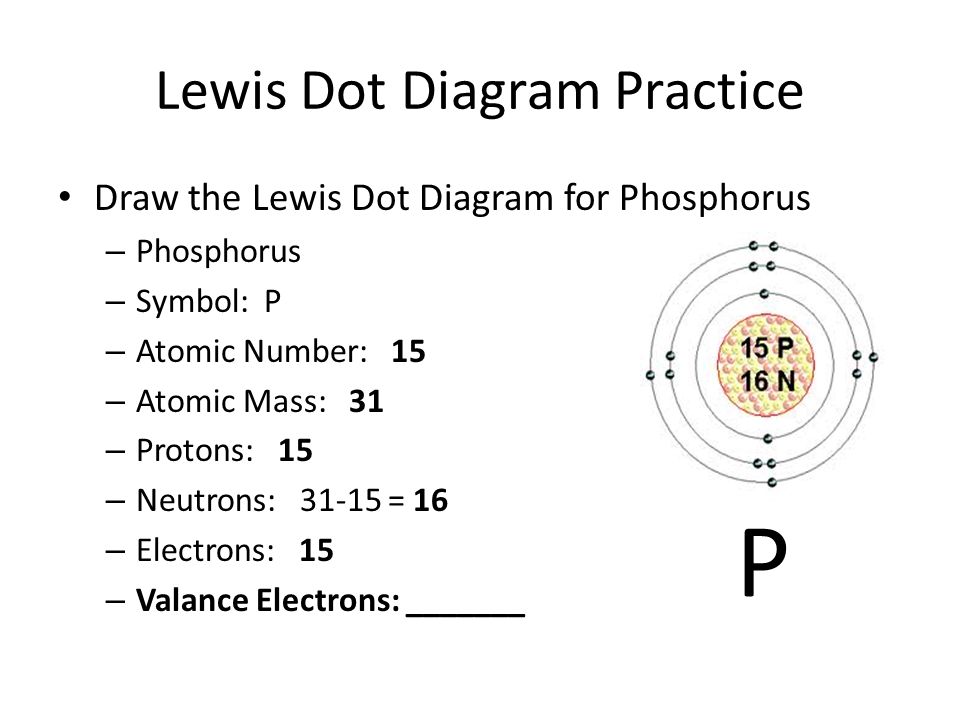
What Is The Number Of Valence Electrons In Phosphorus
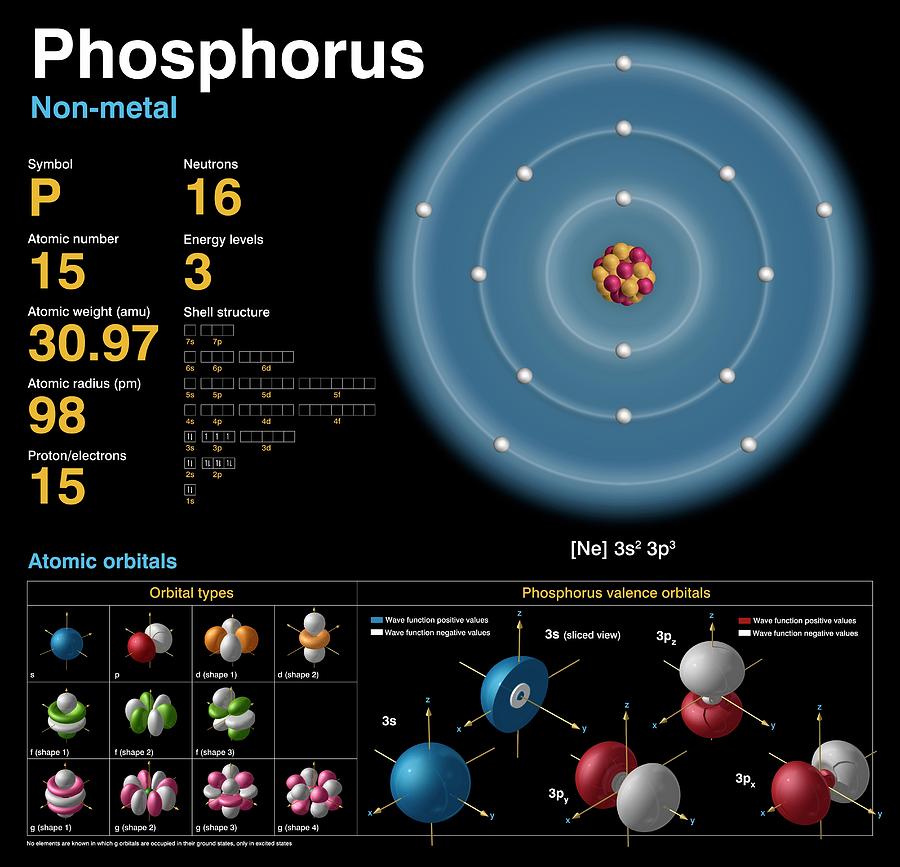
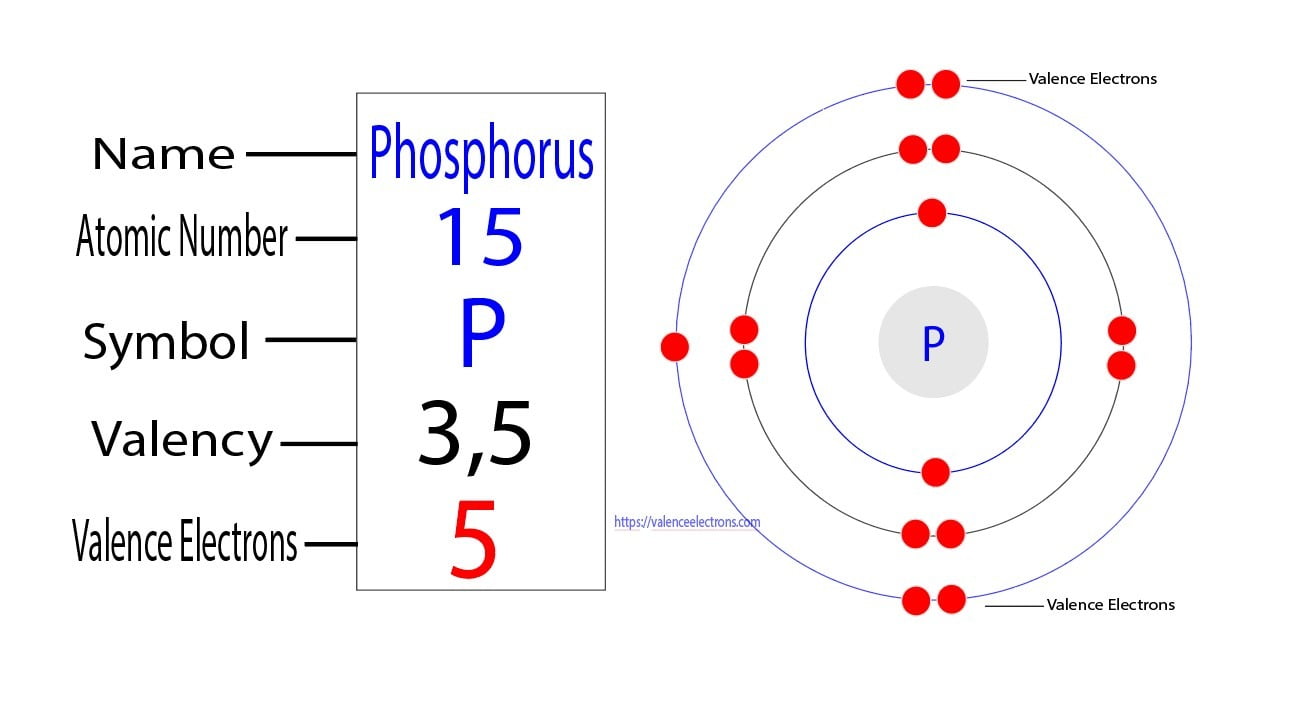
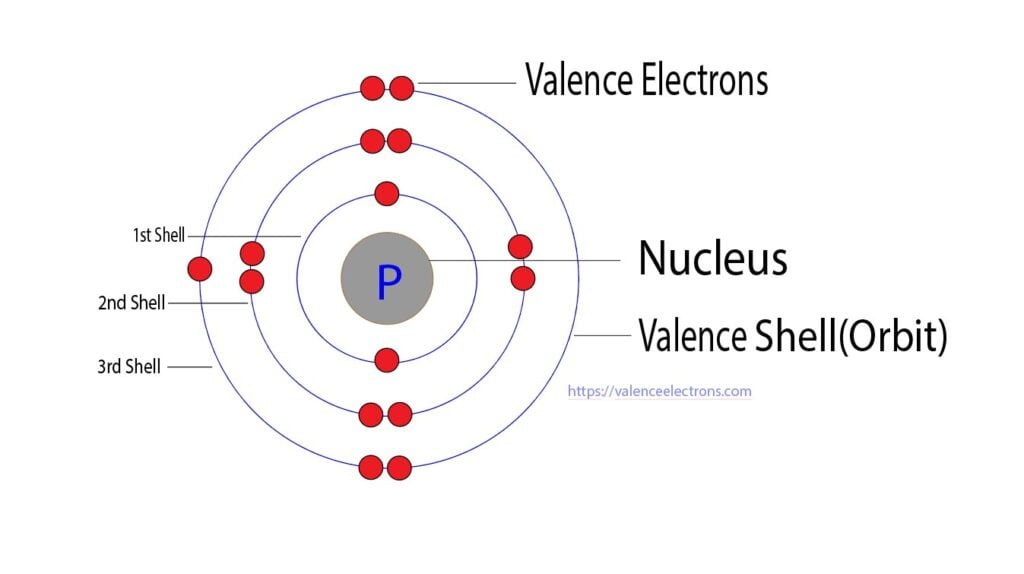
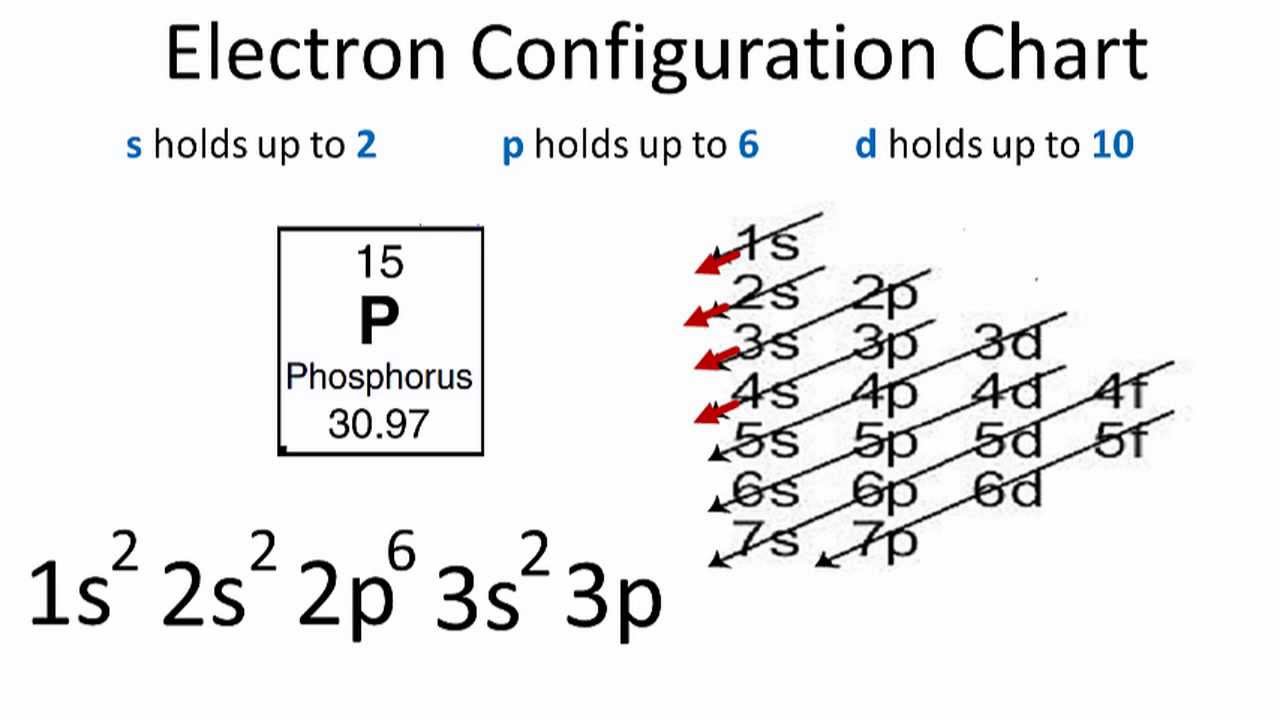
What Is The Number Of Valence Electrons In Phosphorus - It is in period #3# , because it has orbits at. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It has an atomic weight of 30.97376 and a mass. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15.
Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It has an atomic weight of 30.97376 and a mass. It is in period #3# , because it has orbits at.
It has an atomic weight of 30.97376 and a mass. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It is in period #3# , because it has orbits at.
Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
It is in period #3# , because it has orbits at. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. It has an atomic weight of 30.97376 and a mass.
How Many Valence Electrons Does PCl3 Have?
Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It is in period #3# , because it has orbits at. It has an atomic weight of 30.97376 and a mass.
How Many Valence Electrons Does Phosphorus (P) Have?
Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. It has an atomic weight of 30.97376 and a mass. It is in period #3# , because it has orbits at. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table.
Number Of Valence Electrons In Phosphorus cooloup
It is in period #3# , because it has orbits at. It has an atomic weight of 30.97376 and a mass. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15.
Phosphorus Orbital diagram, Electron configuration, and Valence electrons
Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It has an atomic weight of 30.97376 and a mass. It is in period #3# , because it has orbits at. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15.
Phosphorus Electron Configuration (P) with Orbital Diagram
It is in period #3# , because it has orbits at. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It has an atomic weight of 30.97376 and a mass. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15.
Symbol of Phosphorus Archives Dynamic Periodic Table of Elements and
Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It has an atomic weight of 30.97376 and a mass. It is in period #3# , because it has orbits at. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15.
How Many Valence Electrons Are Found In Phosphorus
It is in period #3# , because it has orbits at. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. It has an atomic weight of 30.97376 and a mass. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table.
What Is Phosphorus Atomic Mass at Beverly Belt blog
It is in period #3# , because it has orbits at. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It has an atomic weight of 30.97376 and a mass.
Number Of Valence Electrons In Phosphorus cooloup
It has an atomic weight of 30.97376 and a mass. It is in period #3# , because it has orbits at. Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table.
It Has An Atomic Weight Of 30.97376 And A Mass.
Phosphorus is the 15th element in the periodic table and has a symbol of p and atomic number of 15. Phosphorus has atomic number #15#, and is in group #5# and period #3# of the periodic table. It is in period #3# , because it has orbits at.