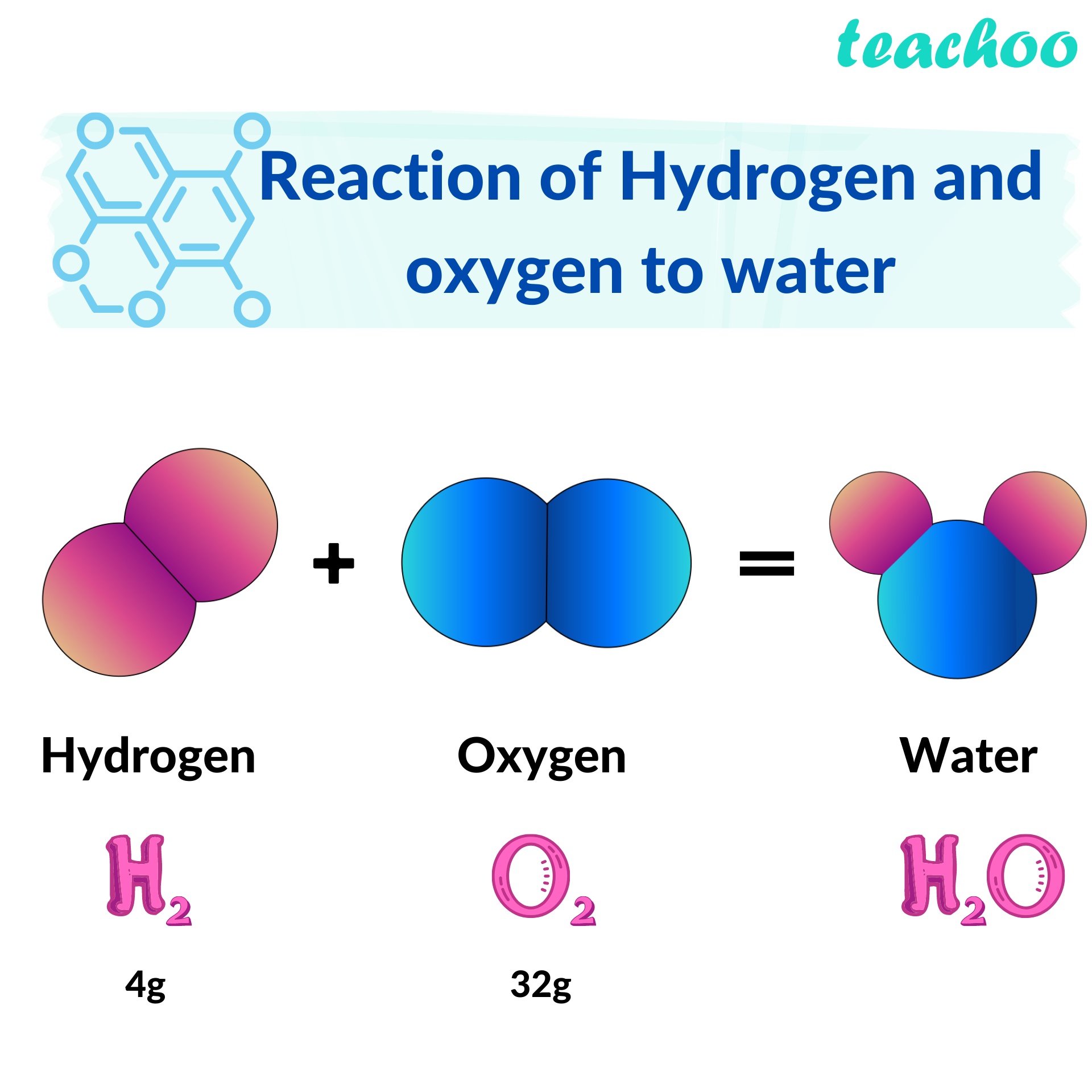
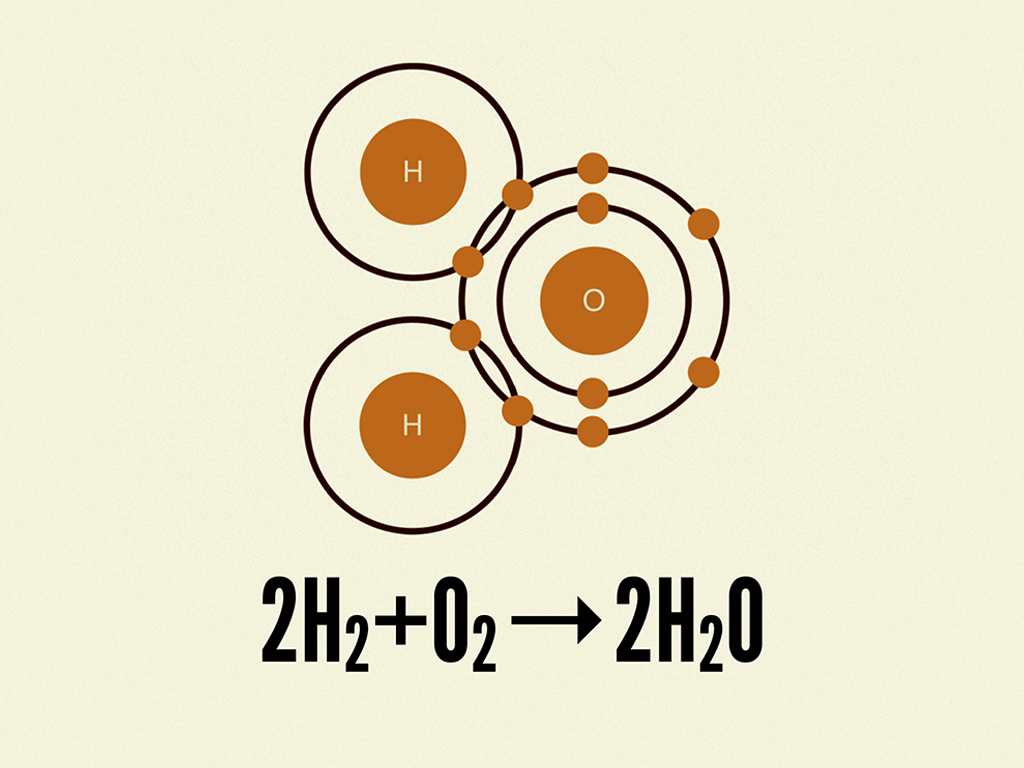
When Hydrogen And Oxygen Combine And Form Water Water Is
When Hydrogen And Oxygen Combine And Form Water Water Is - Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged.
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and.
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged.
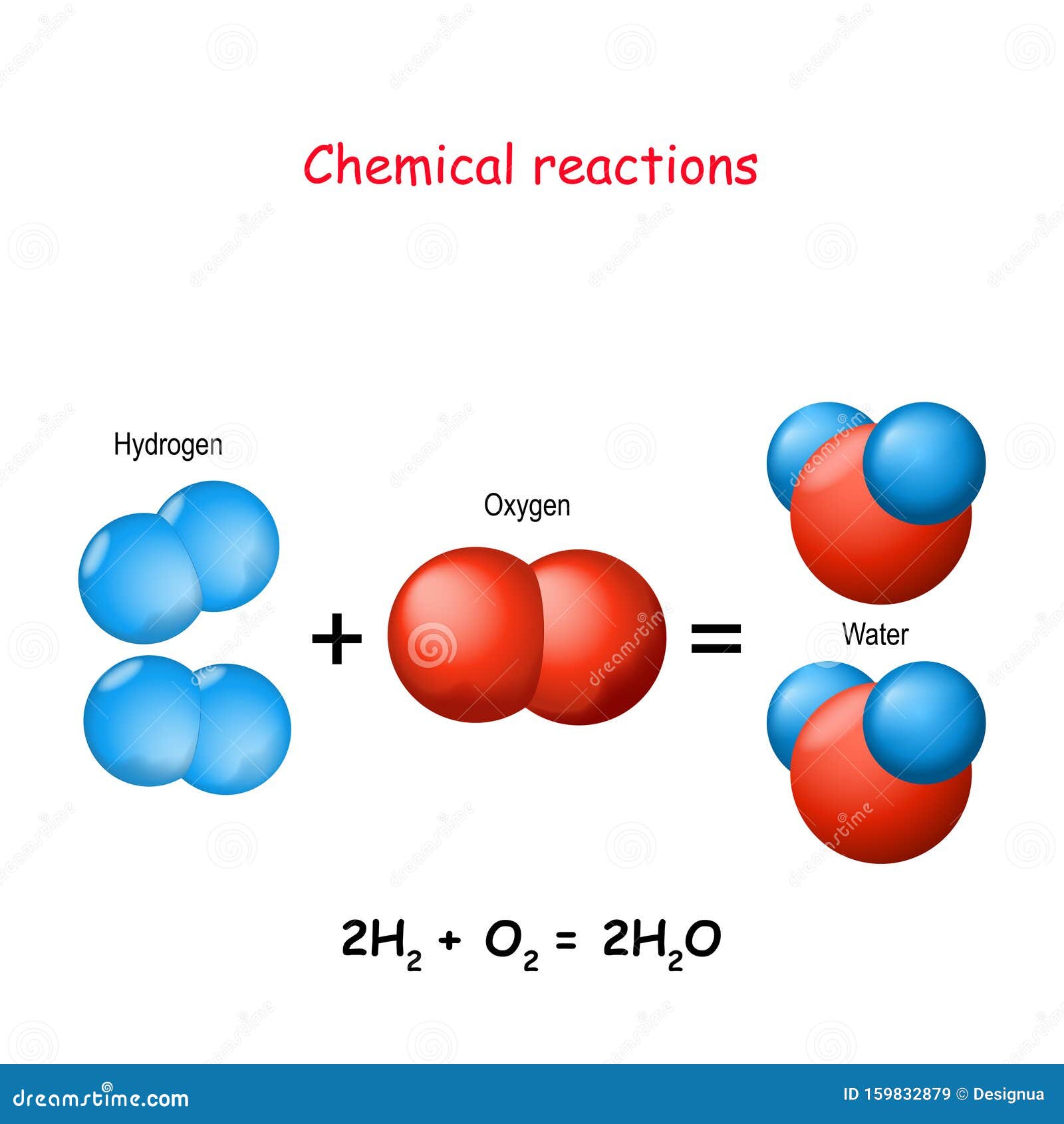
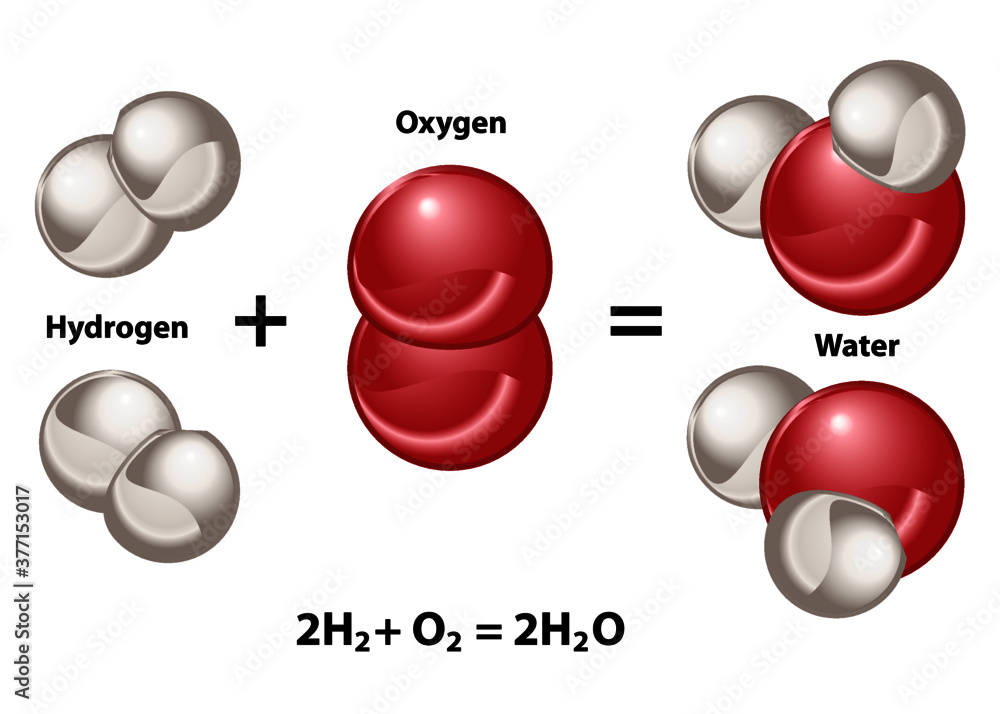
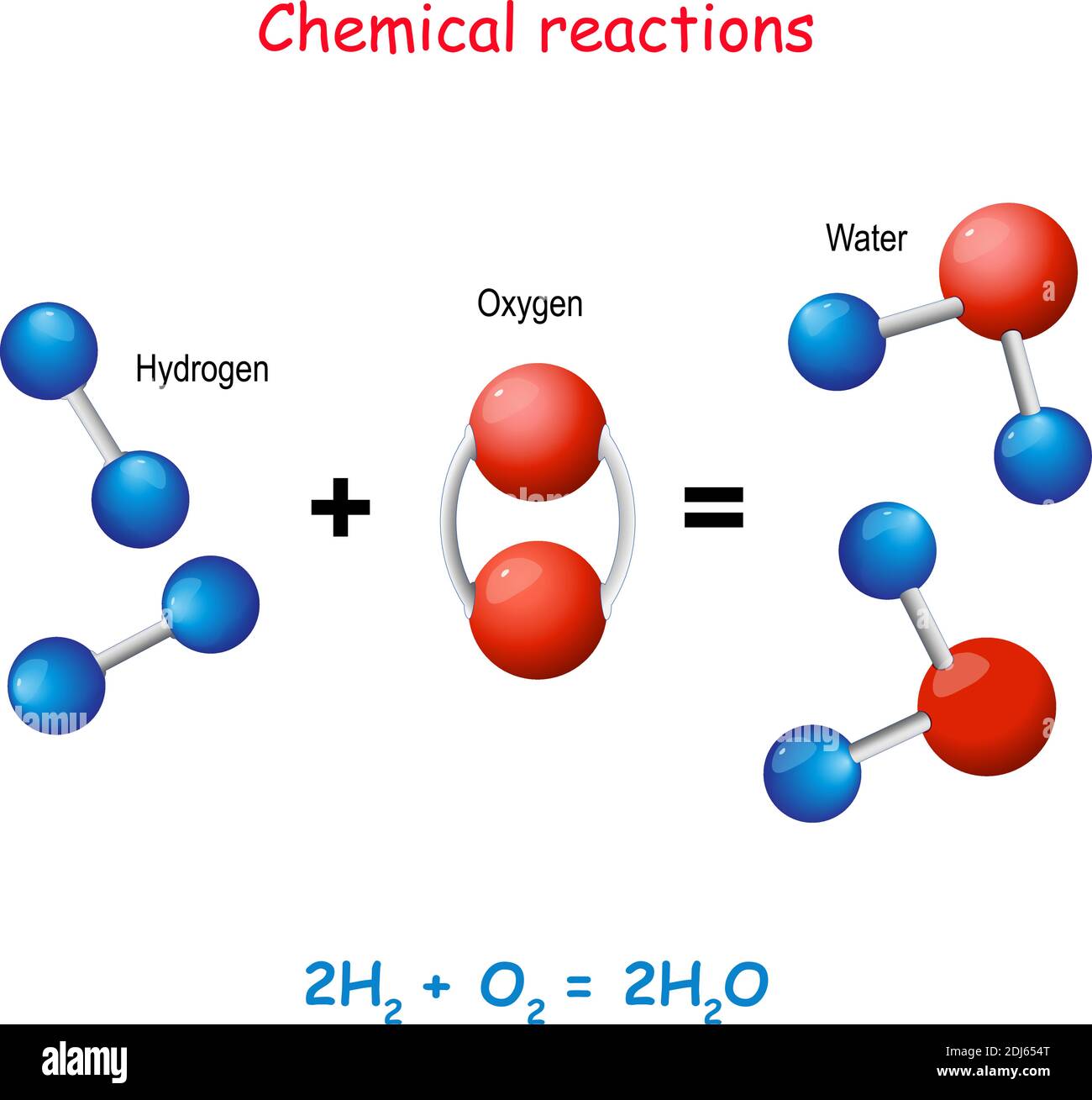
2. Hydrogen and oxygen combine in the ratio of 18 by mass to form water...
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and.
Water Molecule. Oxygen And Hydrogen Cartoon Vector
Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between.
OneClass FIGURE 25 Hydrogen peroxide breaks down into water and
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them.
Compounds Water
While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged.
learning through art water molecules and hydrogen bonding share4uhoshiro
Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between.
[Solved] When hydrogen and oxygen combine to form water, water is
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and.
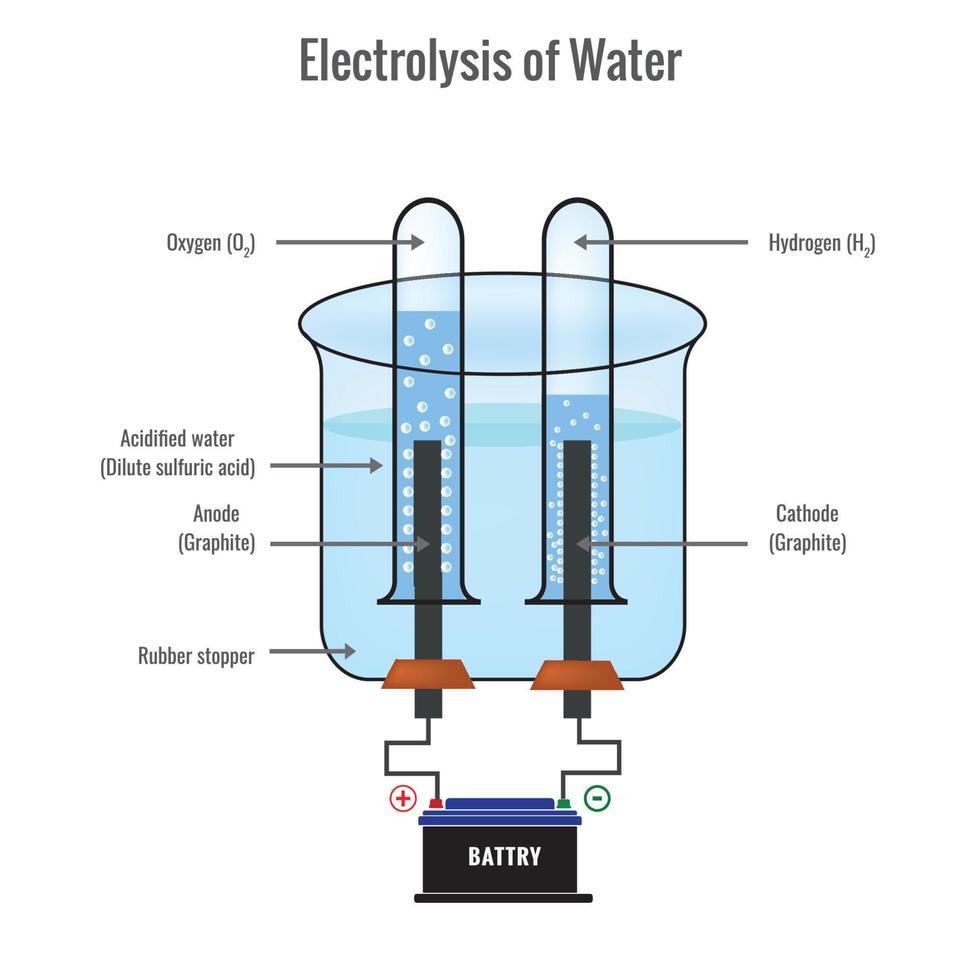
Electrolysis of water forming Hydrogen and Oxygen vector illustration
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of.
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy
Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule.
Hydrogen and oxygen combine in the ratio of 18 by mass to form water
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into.
Atomic Design Methodology Atomic Design by Brad Frost
While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them.
While Water Can Be Formed Through Countless Chemical Reactions, The Most Efficient Way To Create A Water Molecule Out Of Oxygen And.
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen.