Which Pair Of Atoms Can Form An Ionic Bond
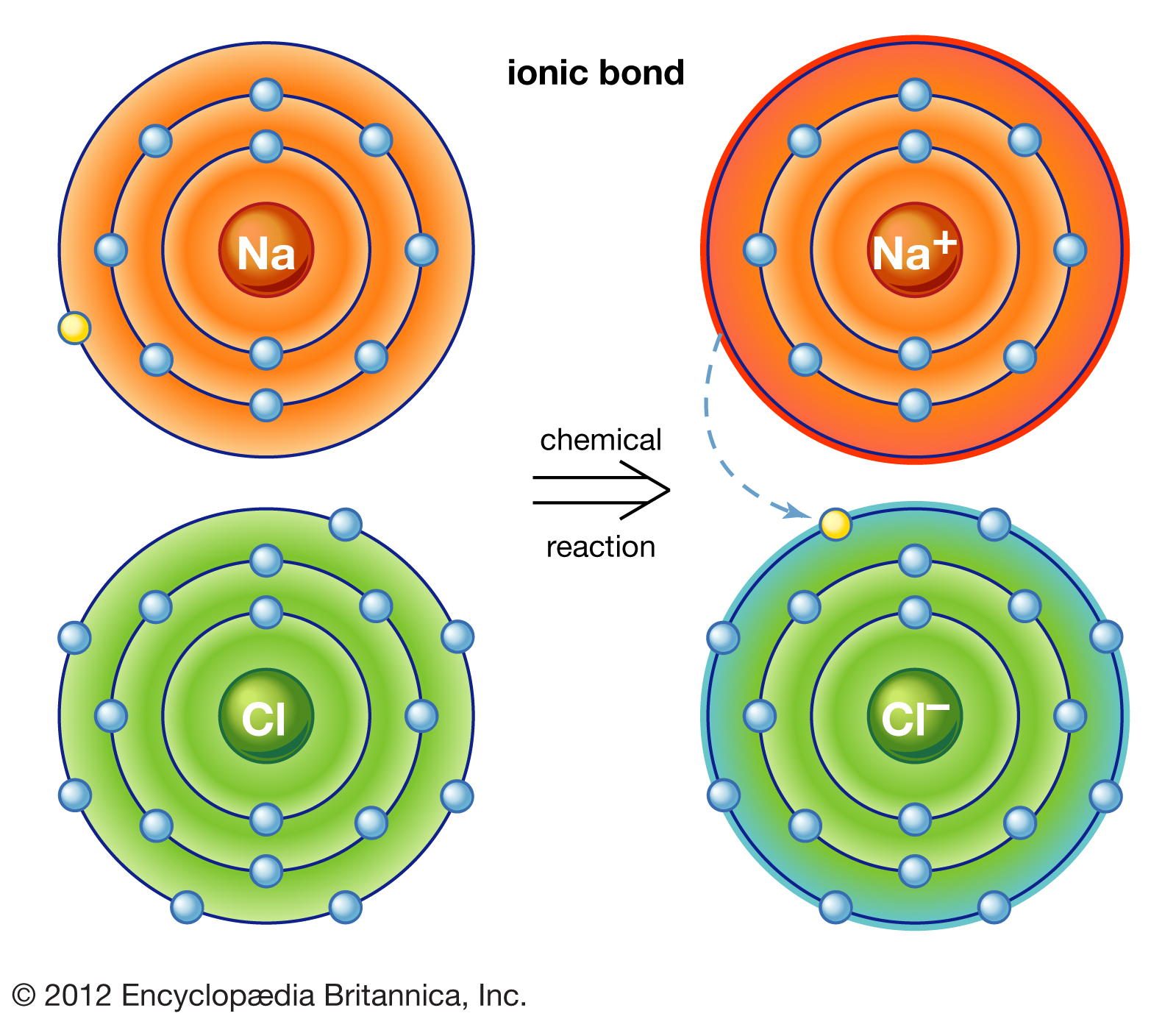
Which Pair Of Atoms Can Form An Ionic Bond - A cation (a positive ion) forms when a neutral atom loses one or more. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces. As you have learned, ions are atoms or molecules bearing an electrical charge. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings.
In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. A cation (a positive ion) forms when a neutral atom loses one or more. As you have learned, ions are atoms or molecules bearing an electrical charge. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces.
A cation (a positive ion) forms when a neutral atom loses one or more. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. As you have learned, ions are atoms or molecules bearing an electrical charge. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces.
What Happens When Two Nitrogen Atoms Share Electrons MarisolkruwLee
In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. A cation (a positive ion) forms when a neutral atom loses one or more. As you have learned, ions are atoms or molecules bearing an electrical charge. Compounds composed of ions are called ionic.
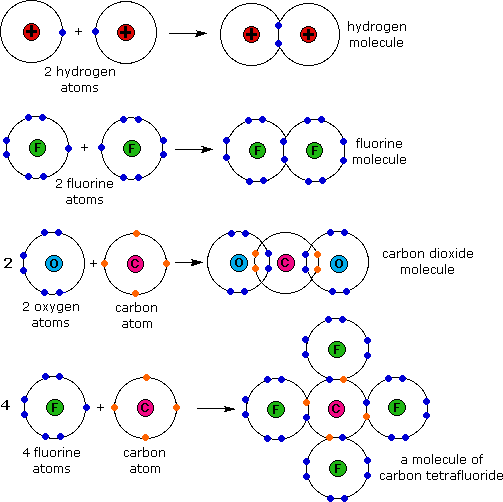
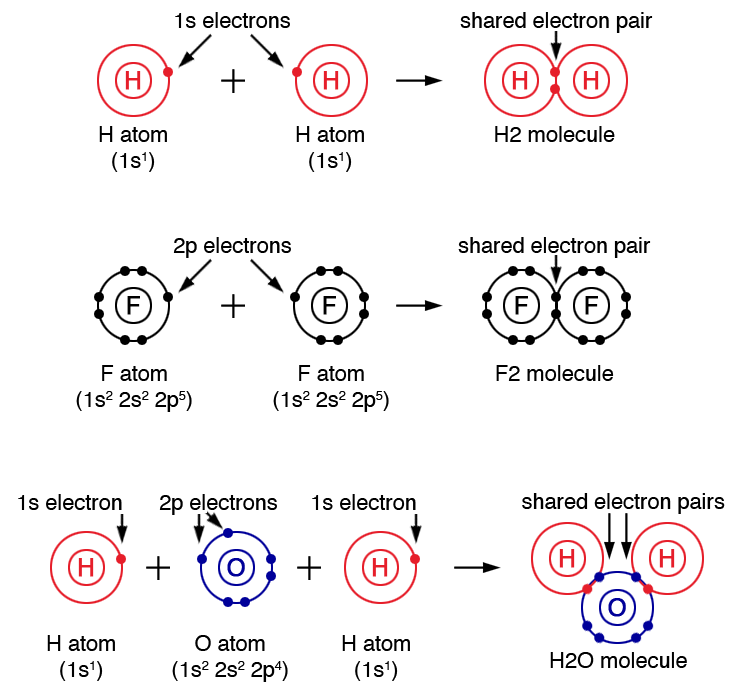
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
A cation (a positive ion) forms when a neutral atom loses one or more. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the.
chemistry knowledge Comparison between Covalent and Ionic Bond
A cation (a positive ion) forms when a neutral atom loses one or more. As you have learned, ions are atoms or molecules bearing an electrical charge. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. Compounds composed of ions are called ionic.
Expanded Electron Configuration of Chlorine Womack Thille
A cation (a positive ion) forms when a neutral atom loses one or more. As you have learned, ions are atoms or molecules bearing an electrical charge. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces. In modern language, the central idea of an ionic bond is.
Atoms of which pair of elements will form ionic bonds in a compound
In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. A cation (a positive ion) forms when a neutral atom loses one or more. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds.
Covalent bonds Learning Lab
As you have learned, ions are atoms or molecules bearing an electrical charge. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. A cation (a positive ion) forms when a neutral atom loses one or more. Compounds composed of ions are called ionic.
What Is An Ionic Bond Sciencing Ionic Bonding Ionic Chemical Bond
Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces. As you have learned, ions are atoms or molecules bearing an electrical charge. A cation (a positive ion) forms when a neutral atom loses one or more. In modern language, the central idea of an ionic bond is.
Ionic Bond — Formation & Compounds Expii
As you have learned, ions are atoms or molecules bearing an electrical charge. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. A cation (a positive ion) forms when a neutral atom loses one or more. Compounds composed of ions are called ionic.
Ionic Bond Definition and Examples
A cation (a positive ion) forms when a neutral atom loses one or more. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. As you have learned, ions are atoms or molecules bearing an electrical charge. Compounds composed of ions are called ionic.
Which pair of atoms form strongest ionic bond? Filo
A cation (a positive ion) forms when a neutral atom loses one or more. As you have learned, ions are atoms or molecules bearing an electrical charge. In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. Compounds composed of ions are called ionic.
A Cation (A Positive Ion) Forms When A Neutral Atom Loses One Or More.
In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds electrostatic forces. As you have learned, ions are atoms or molecules bearing an electrical charge.



.PNG)


